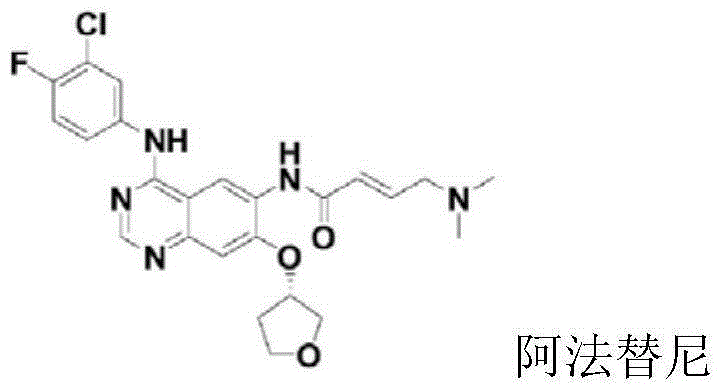
Preparation method for afatinib
A technology of afatinib and a synthetic method, applied in the field of synthesis of heterocyclic compounds, can solve problems such as inability to obtain products, affect stereoselectivity, and unsatisfactory reaction yield, so as to reduce refining processes, improve purity, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
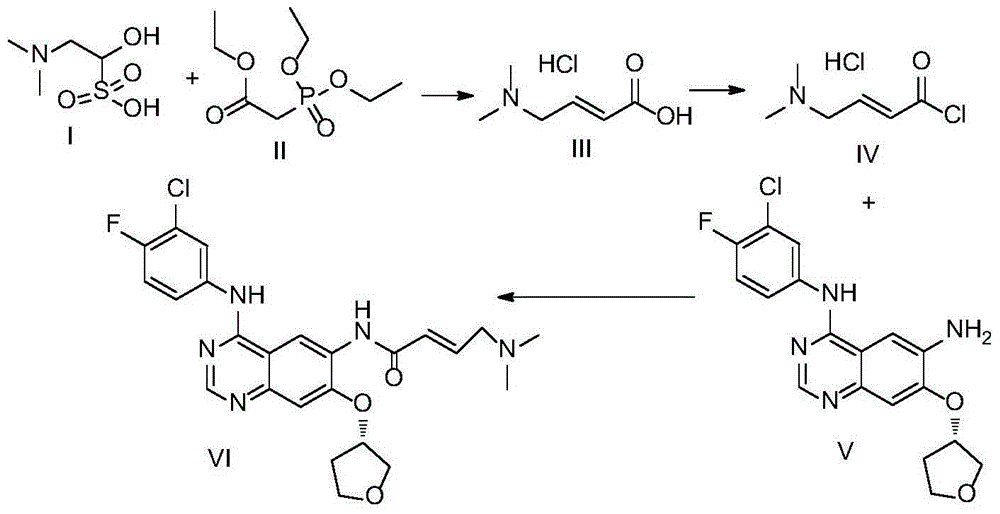
[0024] Embodiment 1~4, the synthesis of trans-4-dimethylamino croton hydrochloride
[0025]
[0026] Operating process:
[0027] 1) Dissolving triethyl phosphonoacetate (II) in solvent 1, the weight to volume ratio of triethyl phosphonoacetate to solvent 1 is 1:A;
[0028] 2) NaOH is added to solvent 2 and stirred evenly, the weight ratio of NaOH to triethyl phosphonoacetate (II) is B, and the weight-to-volume ratio of NaOH to solvent 2 is 1:C, the NaOH dispersed in solvent 2 Slowly add the triethyl phosphonoacetate (II) dissolved in solvent 1 obtained in step 1), and stir for 1 h after the addition is complete to obtain the reaction solution (3), and the reaction temperature is controlled at T1°C;
[0029] 3) Dissolve N,N-dimethylaminoacetaldehyde bisulfite (I) in water and slowly add it dropwise to the reaction solution (3). Descending to obtain the reaction solution (4), the molar ratio of the N,N-dimethylaminoglyoxal bisulfite (I) to triethyl phosphonoacetate (II) is ...
Embodiment 5-1
[0035] Synthesis of Embodiment 5-1 Afatinib (VI)
[0036] The trans-4-dimethylaminocroton hydrochloride (III) prepared by the method of Example 1 was used as raw material, the content was 98.5%, and the feeding amount was 165.6g. The reaction process was as follows:
[0037] Dissolve trans-4-dimethylaminocroton hydrochloride (III) in 1200mL of anhydrous tetrahydrofuran, cool to 0±5°C, add 315mL of oxalyl chloride dropwise, and react at 25±5°C for 4 hours after dropping , and cool the solution to 0±5°C for later use.
[0038] Add 311g of compound (V) (content 99.0%) into 2400mL of anhydrous tetrahydrofuran, cool down to 0±5°C, add the above solution dropwise, and react at 0±5°C until the reaction is detected by TLC. Add 1% sodium hydroxide aqueous solution dropwise to the reaction solution until the pH is 8-9, add 30L of purified water, a large amount of solids are precipitated, filter, wash the solids until the filtered water is neutral, drain and dry to obtain afatinib (VI ...
Embodiment 5-2
[0039] Yield of Afatinib=(Afatinib (VI) quality*content / 485.9) / (Compound (V) quality*0.99 / 374.8) Synthesis of Example 5-2 Afatinib (VI)
[0040] Using the same raw material ratio and process as in Example 5-1, the difference is that ammonia water is used instead of sodium hydroxide aqueous solution to neutralize the reaction solution, the pH is adjusted to 6-7, and 380 g of afatinib (VI) is obtained after washing and drying. The content is 98.5%, and the molar yield based on compound (V) is 93.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com