Combined medicine containing artemisinin
A technology of artemisinin and drugs, applied in the field of combined drugs, can solve problems such as insufficient absorption, less than 80% cure rate of falciparum malaria, serious drug resistance, etc., achieve the effects of reducing toxic side effects, delaying drug resistance, and improving efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
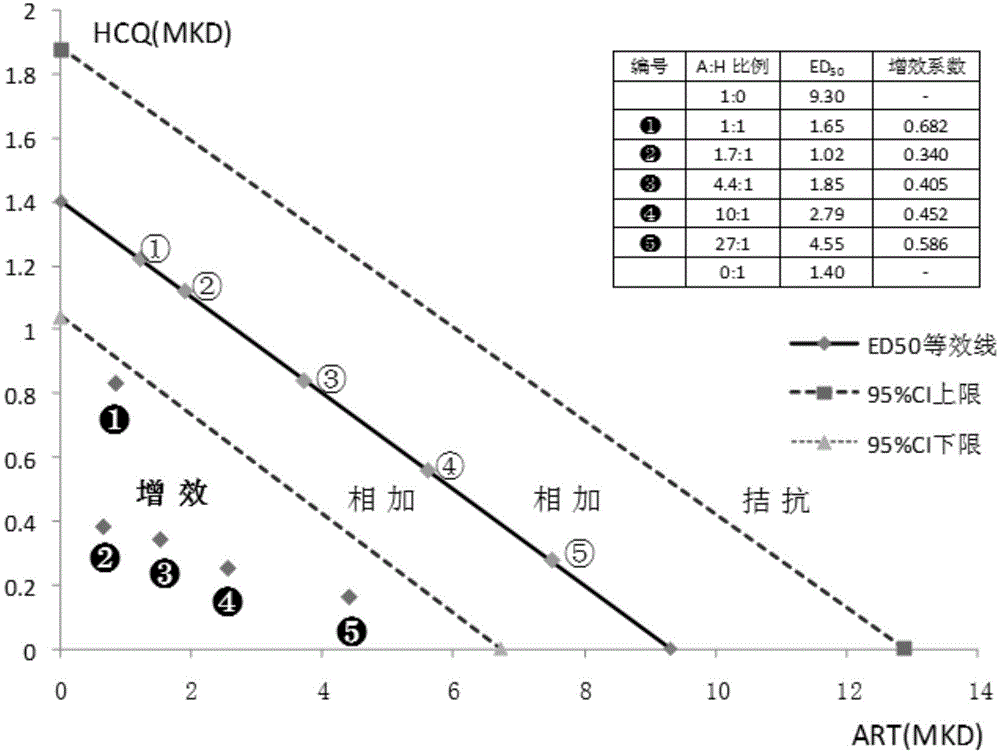
[0021] Experimental example 1 artemisinin and hydroxychloroquine sulfate (hereinafter referred to as AH) compound compatibility synergistic effect
[0022] 1. Materials
[0023] 1.1 Test product
[0024] 1.1.1 Artemisinin (Artemisinin, ART, hereinafter referred to as "A"): Sichuan Tongrentai Pharmaceutical Co., Ltd., batch number: 130602, purity 98.8%;
[0025] 1.1.2 Hydroxychloroquine Sulfate (Hydroxychloroquine Sulfate, HCQ, hereinafter referred to as "H"): Chongqing Kangle Pharmaceutical Co., Ltd., batch number: SQK-130403, purity 98.7%, base content 77.40%.
[0026] 1.1.3 Piperaquine phosphate: Chongqing Kangle Pharmaceutical Co., Ltd., batch number: LPK-100302-M, LPK-111003.
[0027] 1.2 Drug preparation
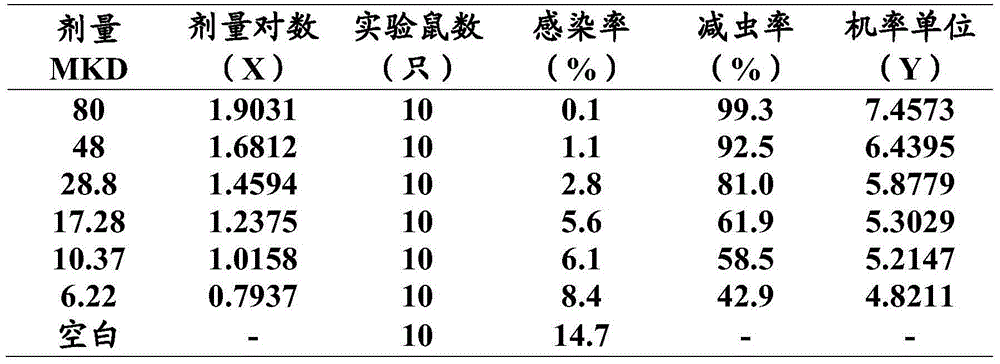
[0028] Use BS210S electronic balance (1 / 10,000) to weigh, add 2 to 3 drops of Tween 80 in a mortar, fully grind to make a suspension, and then dilute with distilled water to the concentration required for the experimental design. Pre-drug preparation. During use, i...
experiment example 2
[0085] Experimental example 2. Drug resistance research of AH compound prescription compatibility
[0086] The 4-day inhibitory effect of AH compound on artemisinin-resistant strains and piperaquine-resistant strains was determined, and whether AH compound had resistance to artemisinin and cross-resistance to piperaquine was evaluated.
[0087] 1. Materials
[0088] Same as Experiment 1
[0089] 2. Test method
[0090] The ED of AH compound was measured by 4-day inhibitory test method 50 / 90 , and with the ED of susceptible strains 50 / 90 Comparing and finding the resistance index I 50 / 90 , with I 50 / 90 To determine the degree of cross-resistance (I 50 / 90 = ED of resistant strain 50 / 90 / susceptible strain ED 50 / 90 ). Its standard is I 90 ≤2 is sensitive; I 90 =2~10 is mild resistance crossover; I 90 =10~100 is moderate resistance cross; I 90 >100 for severe resistance crossover.
[0091] 3. Test results
[0092] Artemisinin single drug and AH compound against ED o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com