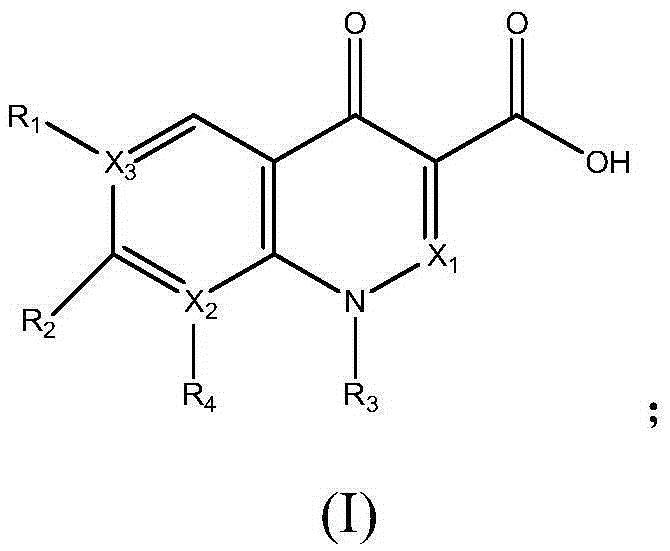
Calcium and zinc heat stabilizer for PVC and application of calcium and zinc heat stabilizer
A technology of heat stabilizer and main stabilizer, applied in the direction of organic chemistry, etc., can solve the problem of easy breeding of various bacteria, achieve good market application prospects, simplify the formula of antibacterial PVC, and reduce costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 pipemidic acid calcium
[0039] Mix 3.033g (8.5mmol) pipemidic acid and 150mL water at room temperature 45°C, add 0.6g (15mmol) sodium hydroxide to a 250mL flask, react at 50°C for 2h to generate pipemidic acid sodium salt, and then add Put 1.11g (10mmol) of calcium chloride into the flask, stir and react at 60°C for 12h, and precipitate occurs. Filter the reaction solution with a Buchner funnel, wash the filter cake with hot water at 70°C until there is no chloride ion in the washing liquid, and place in Dry in a vacuum oven at 30°C for 24 hours to obtain 3.1 g of pipemidic acid calcium.
[0040] Metal elements such as calcium and zinc and fluorine elements were determined by X-ray fluorescence spectrometer (ARLADVANT’X, Thermofisher, USA), and the contents of carbon, hydrogen and nitrogen were determined by organic element analyzer (varioMacrocube, Elementar, Germany).
[0041] Elemental analysis results: 6% calcium; 52% carbon; 22% ni...
Embodiment 2
[0042] Embodiment 2 zinc pipemidate
[0043] Mix 3.033g of pipemidic acid (8.5mmol) with 150mL of water at 45°C, add 0.6g (15mmol) of sodium hydroxide in a 250mL flask, and react at 40°C for 1h to generate pipemidic acid sodium salt, then add 2.745 Add g (15mmol) zinc acetate to the flask, continue to stir and react for 4h, and precipitate occurs. The reaction solution is filtered with a Buchner funnel, and the filter cake is washed with hot water at 70°C until there is no acetate ion in the washing liquid, and placed in a vacuum oven at 40°C. After drying for 24 hours, 2.9 g of zinc pipemidate was obtained.
[0044] The element test method is the same as in Example 1.
[0045] Elemental analysis results: 10% zinc; 50% carbon; 20% nitrogen; 6% hydrogen.
Embodiment 3
[0046] Embodiment 3 norfloxacin zinc
[0047] Mix 3.192g (10mmol) of norfloxacin with 190mL of distilled water at 60°C, add 2.12g (20mmol) of sodium carbonate, react at 30°C for 2h, add 1.45g (9mmol) of zinc sulfate, and stir for 6h to produce Precipitate, filter the reaction solution with a Buchner funnel, wash the filter cake with hot water at 70°C until there is no sulfate ion in the washing solution, and dry it in vacuum at 50°C for 12 hours to obtain 3.1 g of norfloxacin zinc.
[0048] The element test method is the same as in Example 1.
[0049] Elemental analysis results: 9% zinc; 5% fluorine; 55% carbon; 12% nitrogen; 6% hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com