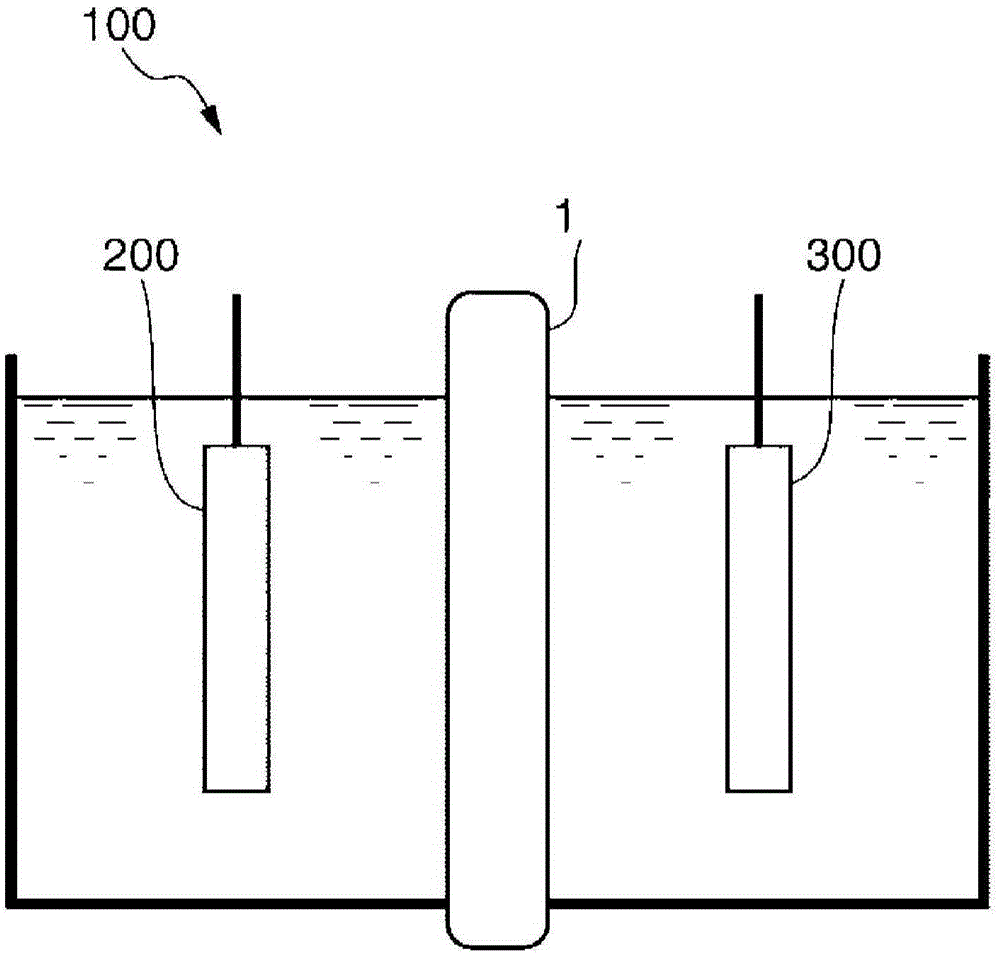
Fluorine-containing polymer, cation exchange membrane, and electrolysis vessel
A cation exchange membrane, ion exchange technology, applied in cation exchange materials, electrolysis components, electrolysis process, etc., can solve the problem of reducing current efficiency and achieve the effect of stabilizing current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] (Production of fluorine-containing polymers)
[0153] Solution polymerization was performed in order to obtain a carboxylic acid type fluorine-containing polymer. As the stirring blade, an anchor type stirring blade was used. First, add CF to a stainless steel 20L autoclave 2 = CFOCF 2 CF(CF 3 )O(CF 2 ) 2 COOCH 3 5160g, HFC-43-10mee15633g, after fully replacing the container with nitrogen, further use CF 2 = CF 2 (TFE) to replace, heated until the temperature inside the container became stable at 35°C, and pressurized to 0.232 MPa-G (gauge pressure) with TFE. Next, (CF 3 CF 2 CF 2 COO) 2 5% HFC43-10mee solution 103g, methanol 2.04g as chain transfer agent, start the reaction. TFE was fed intermittently while stirring at 35°C, and 1.86 g of methanol was added on the way to lower the TFE pressure from 0.232 MPa-G at the initial stage to 0.206 MPa-G at the end, and the polymerization was stopped when 688 g of TFE was supplied. After discharging the unreacted ...
Embodiment 2
[0166] In order to produce a fluorine-containing polymer having a different melt index a, in Example 1, the amount of methanol added initially was changed to 0.22 g, and the amount of methanol added midway was changed to 0.20 g, and the procedure was carried out in the same manner as in Example 1. Polymerization to obtain a carboxylic acid type fluorine-containing polymer. The obtained fluorine-containing polymer was pelletized with a twin-screw extruder. The EW of the fluorine-containing polymer was 1122 g / eq.
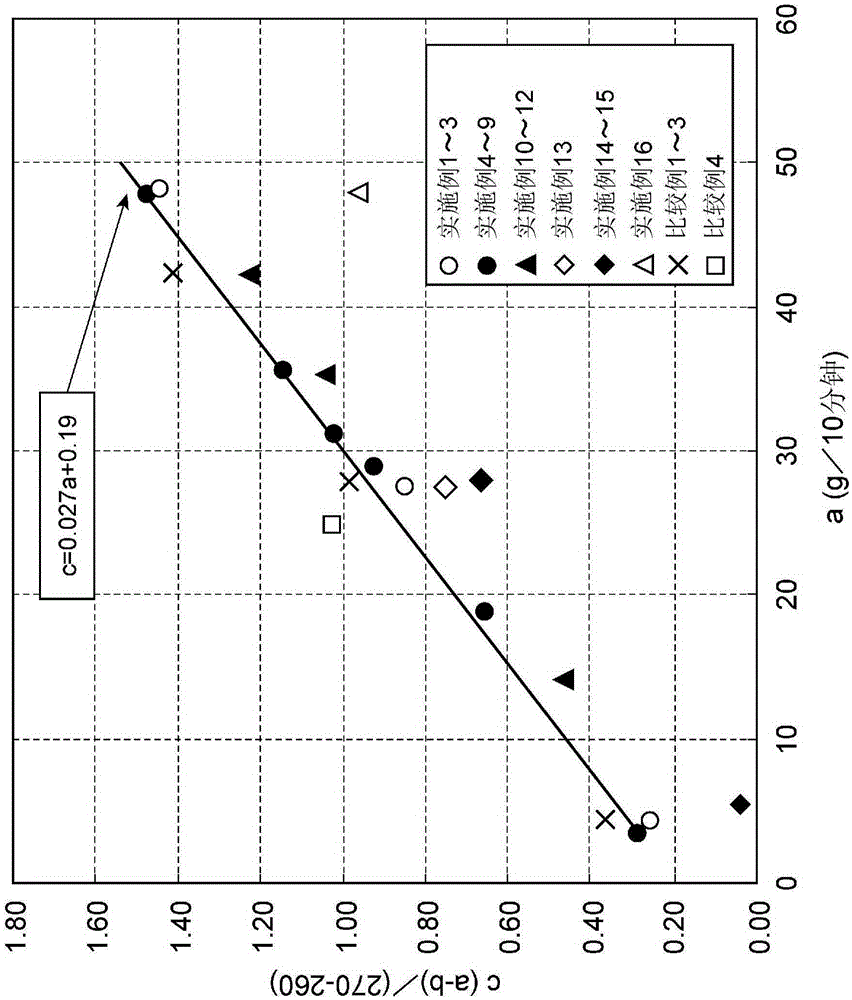
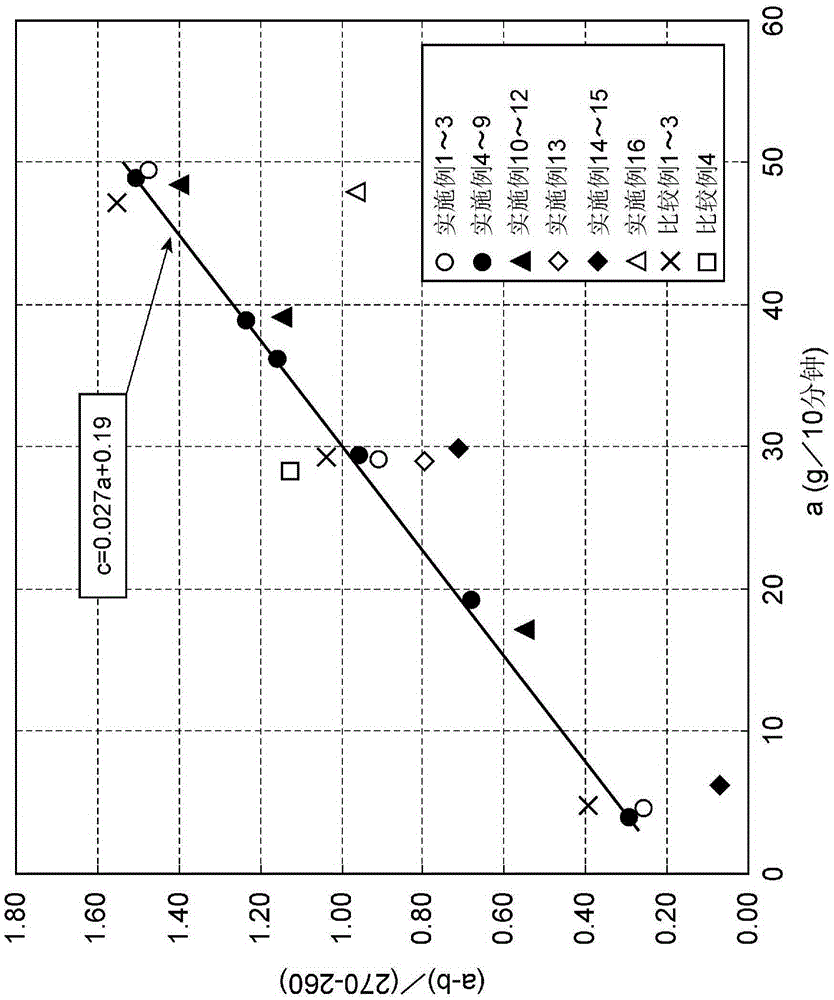
[0167] The melt index a of the carboxylic acid type fluorine-containing polymer was 4.35 (g / 10 minutes), and the melt index b was 1.80 (g / 10 minutes). In addition, using a and b to calculate c, the result is 0.26, c≤0.027×a+0.19. The result of plotting the vertical axis c and the horizontal axis a is shown in figure 2 .
[0168] A cation exchange membrane was produced in the same manner as in Example 1 except that the above-mentioned carboxylic acid type fluorine...
Embodiment 3
[0172] In order to produce a fluorine-containing polymer having a different melt index a, in Example 1, the amount of methanol added initially was changed to 1.62 g, and the amount of methanol added midway was changed to 1.47 g, and the procedure was carried out in the same manner as in Example 1. Polymerization to obtain a carboxylic acid type fluorine-containing polymer. The obtained fluorine-containing polymer was pelletized with a twin-screw extruder. The EW of the fluorine-containing polymer was 1119 g / eq.
[0173] The melt index a of the carboxylic acid type fluorine-containing polymer was 27.56 (g / 10 minutes), and the melt index b was 19.07 (g / 10 minutes). In addition, using a and b to calculate c, the result is 0.85, c≤0.027×a+0.19. The result of plotting the vertical axis c and the horizontal axis a is shown in figure 2 .
[0174] A cation exchange membrane was produced in the same manner as in Example 1 except that the above-mentioned carboxylic acid type fluori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com