A chemically modified nucleic acid aptamer as1411 and its use
A technology of AS1411 and nucleic acid aptamer, which is applied in the direction of medical preparations containing active ingredients, scientific instruments, pharmaceutical formulas, etc., can solve the problems of shortened action distance, increased action distance, and increased activity, so as to improve binding force and increase Inhibition, enhance the effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 The solid-phase synthesis of AS1411 modified by isonucleoside or isonucleoside combined with 2'-deoxyinosine
[0040] DNA was synthesized using an Appllied Biosystems model 394 DNA solid-phase synthesizer.
[0041] Normal deoxynucleoside phosphorylation monomers (dT, dGAc, dABz, dCAc) were purchased from Shanghai Jima Pharmaceutical Technology Co., Ltd.; 2'-deoxyinosine phosphoramidite monomers (compound VI) were purchased from Shanghai Zhiyan Technology Co., Ltd. Co., Ltd. (Shanghai) purchased. CPG (CPG-dG), CAP-A and CAP-B, oxidized I2 solution, Cl 3 CCOOH was purchased from Beijing Aoke Biotechnology Company; 0.25M 5-Ethylmercapto 1H-tetrazolium solution was purchased from Shanghai Zhiyan Technology Co., Ltd. (Shanghai).
[0042] According to the method of literature (HW Yu, LR Zhang, JC Zhuo, LT Ma, LH Zhang, Bioorg.Med.Chem., 1996,40,609-614), the isonucleoside compound shown in chemical formula I and chemical formula II is prepared respectively The is...
Embodiment 2
[0058] Research on the basic properties of the modified AS1411 sequence in Example 2
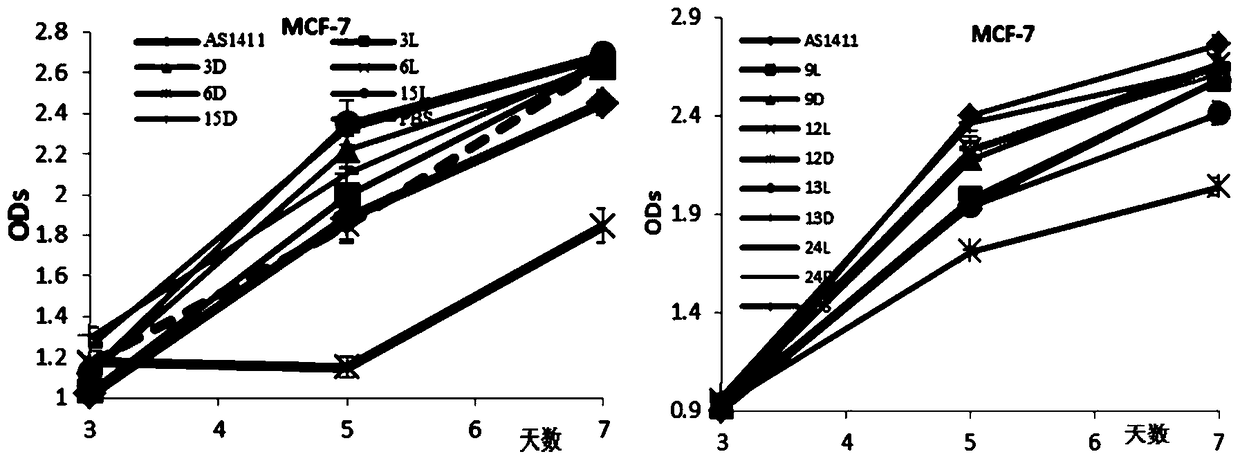
[0059] 1. Sample name: The 3rd, 6th, 9th, 12th, 13th, 15th or 24th position of the AS1411 sequence is mixed with the isonucleoside shown in chemical formula I or chemical formula II (wherein Base is selected from thymine T) , the modified AS1411 sequence obtained by coupling the corresponding positions instead of natural nucleosides, prepared by the method in Example 1.
[0060] 2. CD spectral analysis
[0061] The AS1411 sequence samples were dissolved in PBS, and all samples were denatured at 95°C for 5 minutes, then slowly lowered to room temperature, and then tested. The CD scanning range is from 200-400nm, the temperature is constant at 25°C, the scanning interval is 0.2nm, and the scanning speed is 100nm / min. Each sample is scanned for 3 times, and the instrument's own software is used for smoothing. See the experimental results figure 1 .
Embodiment 3
[0062] Example 3 SPR determination of isonucleoside-modified and isonucleoside combined deoxyinosine modified AS1411 sequence and nucleolin affinity
[0063] 1. Sample name: AS1411-6L, AS1411-12D, AS1411-13L, AS1411-6L / 12D, AS1411-6L / 12D / 24dI, prepared by the method in Example 1.
[0064] 2. Method
[0065] (1) The nucleolin protein was immobilized on the CM5 chip.
[0066] A certain amount of nucleolin protein was prepared into a series of solutions with appropriate pH value of sodium acetate solution, and the optimal coupling conditions of the protein on the surface of the chip were optimized. The specific operation is as follows: PBS buffer is the mobile working solution, the temperature is 25°C, the flow rate is 10 μL / min, and the baseline is stabilized. The mixture of EDC and NHS was flowed over the surface of the chip at a flow rate of 5 μL / min for 7 minutes to activate the carboxyl groups on the surface. Nucleolin protein solutions of different concentrations were pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com