Application of L-ascorbyl glycoside compounds to preparation of alpha-glucosidase inhibitors
A technology of glucosidase and acid-based glycosides, which is applied in the field of L-ascorbic acid-based glycosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
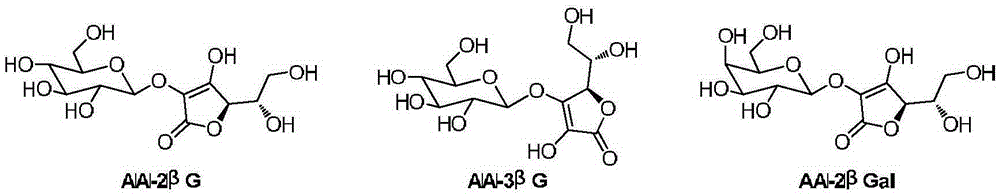
[0009] Preparation of 2-O-β-D-glucosyl-L-ascorbic acid
[0010] 3-O-benzyl-5,6-O-isopropylidene ascorbic acid (3.06g, 10mmol) and tetraacetylbromoglucose (4.94g, 12mmol) were added to 60mL of dichloromethane and water mixed solvent, Then add K 2 CO 3 (1.66g, 12mmol) and TBABr (12mmol), stirred at room temperature for 5 hours. Separate the liquid, extract the aqueous phase once with dichloromethane, combine the organic phases, wash twice with citric acid solution, then wash once with saturated brine, dry over anhydrous magnesium sulfate, filter, spin the filtrate, and separate by column chromatography 2-O-β-D-tetraacetylglucosyl-3-O-benzyl-L-ascorbic acid (5.09 g, 80%) was obtained.
[0011] 2-O-β-D-tetraacetylglucosyl-3-O-benzyl-L-ascorbic acid (2g, 3.14mmol) was dissolved in 50mL aqueous acetic acid, and reacted at 80°C for 1 hour. After cooling, 50 mL of ethyl acetate was added to the reaction system, washed with water and saturated sodium bicarbonate solution in turn, t...
Embodiment 2
[0014] Preparation of 3-O-β-D-glucosyl-L-ascorbic acid
[0015] 5,6-O-Isopropylidene ascorbic acid (2.00 g, 9.26 mmol) was added to 60 mL of DMF, followed by K 2 CO 3 (1.92g, 14mmol) and tetraacetylbromoglucose (5.71g, 14mmol), stirred at room temperature for 10 hours. Stop the reaction, extract the reaction system with ethyl acetate, wash the organic phase successively with citric acid solution and saturated brine, then dry with anhydrous magnesium sulfate, filter, spin the filtrate to dry, and separate by column chromatography to obtain 3-O-β-D - Tetraacetylglucosyl-L-5,6-O-isopropylidene ascorbic acid (3.31 g, 65%).
[0016] Add 65 mL of acetic acid solution to 3-O-β-D-tetraacetylglucosyl-L-5,6-O-isopropylidene ascorbic acid (1.05 g), and react at 80° C. for 2.5 h. The reaction was stopped, and 50 mL of ethyl acetate was added to the reaction system to extract the product, and the liquid was separated, and the organic phase was followed by water, saturated NaHCO 3 Wash ...
Embodiment 3
[0019] Preparation of 2-O-β-D-galactosyl-L-ascorbic acid
[0020] Add 3-O-benzyl-5,6-O-isopropylidene ascorbic acid (2.45 g, 8 mmol) and tetraacetylbromogalactose (3.95 g, 9.6 mmol) to 50 mL of 60 mL dichloromethane and water mixed solvent , under uniform stirring at room temperature, add K 2 CO 3 (1.33g, 9.6mmol) and TBABr (12mmol), continue stirring for 5 hours. Separate the liquid, extract the aqueous phase once with dichloromethane, combine the organic phases, wash twice with citric acid solution, then wash once with saturated brine, dry with anhydrous magnesium sulfate, filter, spin the filtrate, and perform column chromatography 2-O-β-D-tetraacetylglucosyl-3-O-benzyl-L-ascorbic acid (3.87 g, 76%) was isolated.
[0021] 2-O-β-D-tetraacetylglucosyl-3-O-benzyl-L-ascorbic acid (1.91g, 3mmol) was dissolved in 50mL aqueous acetic acid, and reacted at 80°C for 1 hour. After cooling, 50 mL of ethyl acetate was added to the reaction system, washed with water and saturated sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com