Triptorelin slow-release micro particles and preparation method thereof
A technology of slow-release microparticles and microparticles, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Low-level problems, to achieve good plasticity, simple process, good drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
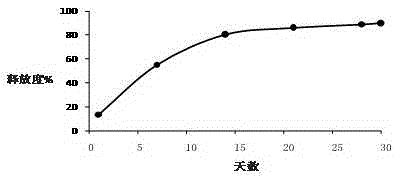
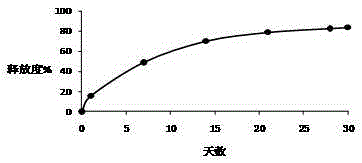
[0026] Example 1-3: Microparticles were prepared according to different types of PLGA, and the release time, drug loading and encapsulation efficiency of the prepared triptorelin microparticles were investigated under the same preparation conditions.
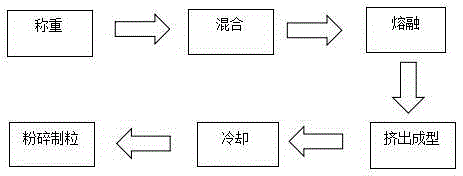
[0027] Weigh 50 mg of triptorelin (4.572%), 1 g of PLGA (95.048%) (see Table 1 for the model of PLGA, different models represent different mass ratios of lactic acid and glycolic acid), poloxamer 1882.1 mg (0.2%), Feed into the feeding hopper, set the feeding hopper speed to 120 rpm, hot-melt extrusion temperature to 80°C, hot-melt extrusion time to 20 minutes, crushing and cooling temperature after extrusion to -90°C, collect the crushed particles, and detect Physical and chemical properties. The test results are shown in Table 1 below.
[0028] Table 1 Microparticles prepared by different types of PLGA
[0029] Example PLGA model Drug loading encapsulation rate release time Example 1 85:15 PLGA 6.4%...
Embodiment 4-6
[0030] Example 4-6: Microparticles were prepared according to different amounts of PLGA, and under the same preparation conditions, the release time, drug loading and encapsulation efficiency of the prepared triptorelin microparticles were investigated.
[0031]Take triptorelin 50mg, different amounts of PLGA of 75:25 type, poloxamer 1880.4%, send into the feeding funnel, set the feeding funnel rotating speed as 120 rev / min, hot melt extrusion temperature is 80 ℃, heat The melt-extrusion time was 20 minutes, and the pulverization cooling temperature after extrusion was -90°C. The pulverized particles were collected and their physical and chemical properties were tested. The test results are shown in Table 2 below.
[0032] Table 2 Microparticles prepared with different triptorelin and PLGA ratios
[0033] Example Triptorelin PLGA Drug loading encapsulation rate Example 4 50mg (7.66%) 0.6g(91.94%) 6.2% 74.1% Example 5 50mg (5.86%) 0.8g(93.74...
Embodiment 7-15
[0034] Example 7-15: 9 parts of materials were weighed with the prescription ratio of 4.5% triptorelin, 95% PLGA, and 0.5% poloxamer, and prepared by combining the process parameters in the following table 3 respectively. Microparticles were tested for encapsulation efficiency, drug loading, and release time.
[0035] Table 3 Microparticles prepared by different process parameters
[0036]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com