Induced differentiation method for differentiating human induced pluripotent stem cells into leydig cells and application thereof
A technology of Leydig cells and pluripotent stem cells, which is applied in the field of induced differentiation of human induced pluripotent stem cells to Leydig cells, can solve the problems of unknown origin of LC development, difficulty in obtaining materials, and difficulty in expansion, and achieves the goal of improving serum The effect of testosterone levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Differentiation from iPS cell lines to hNCSCs
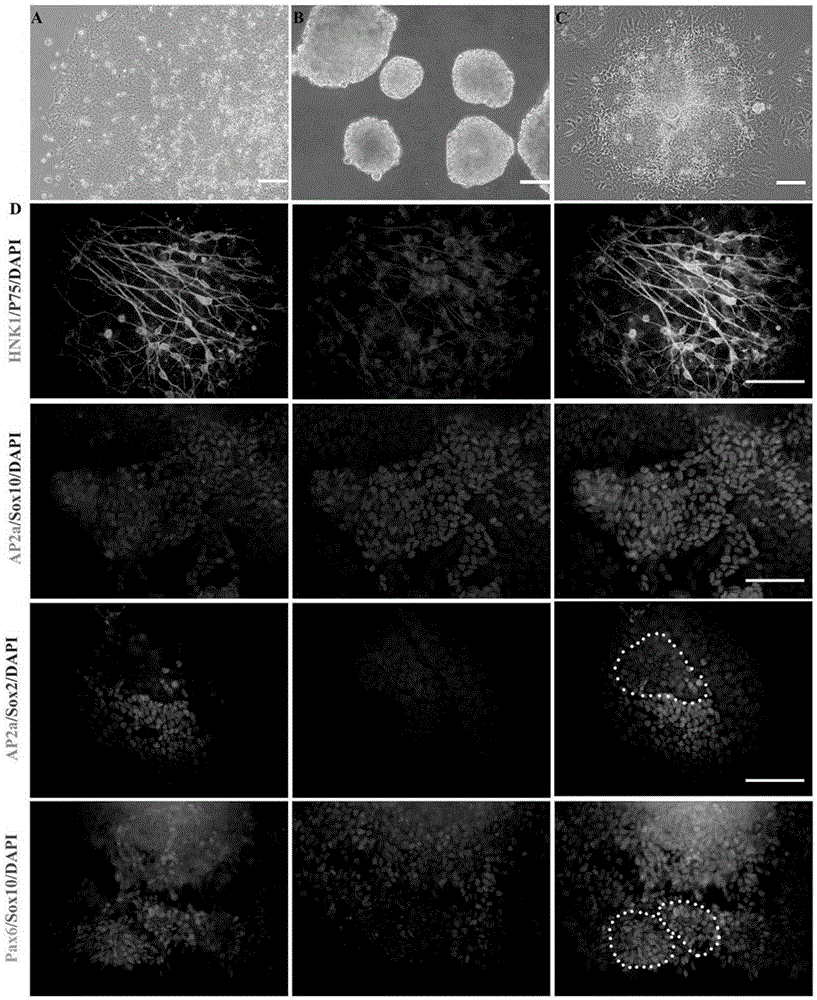
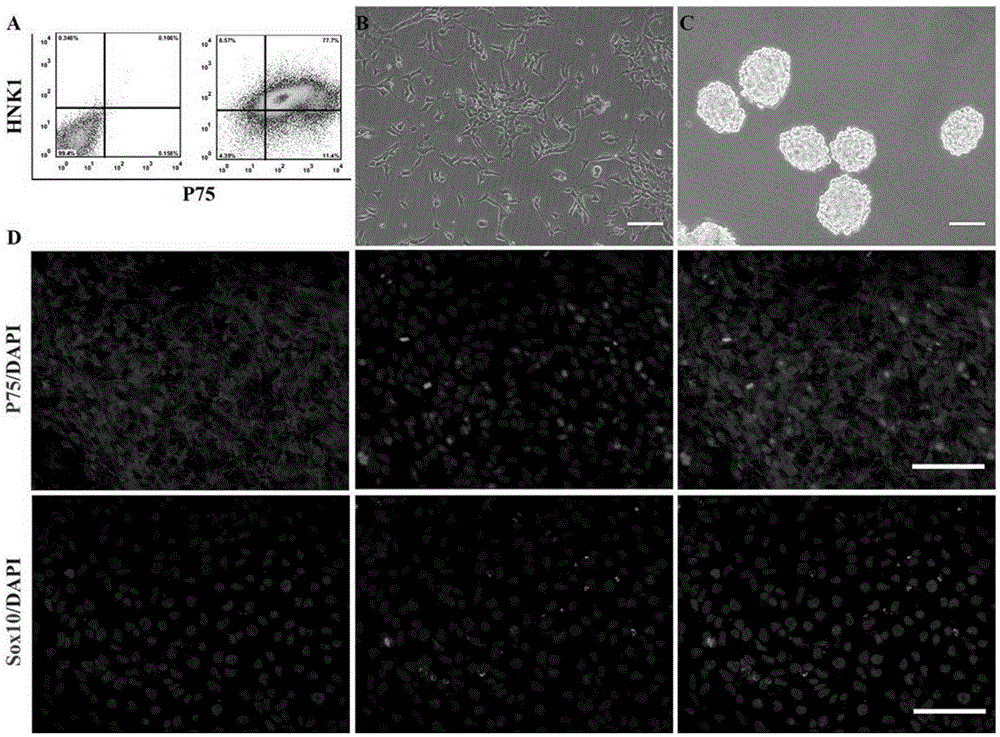
[0048] 1) Preparation of cell suspension: using the established human iPS cell line (hiPSCs, HumMolGenet.2013, 22(11):2221-33), hiPSCs cells grow in flat clones when expanded and cultured on Matrigel, and the cells are arranged close, such as figure 1 As shown in A. The hiPSCs cells were digested into small pieces with 0.5 mmol / L EDTA, and the cells were resuspended in mTeSR medium containing ROCK inhibitor. The ROCK inhibitor used therein was Y27632 (Calbiochem, San Diego, CA).
[0049] 2) Induce differentiation: collect suspension cells, inoculate in Petri dishes, use neural differentiation medium (80% Knockout TM DMEM, 18% Knockout TM SR, 1% (V / V) penicillin-streptomycin mixed solution, 1mML-glutamic acid, 0.1mM β-mercaptoethanol) suspension culture to form translucent spherical embryo bodies, such as figure 1 Shown in B. where Knockout TM DMEM and Knockout TM SRs were purchased from Invitrogen, Carlsb...
Embodiment 2
[0056] Example 2 Induction of differentiation from hNCSCs into Leydig cells (iPS-hNCSCs-LCs or hNCSCs-LCs)
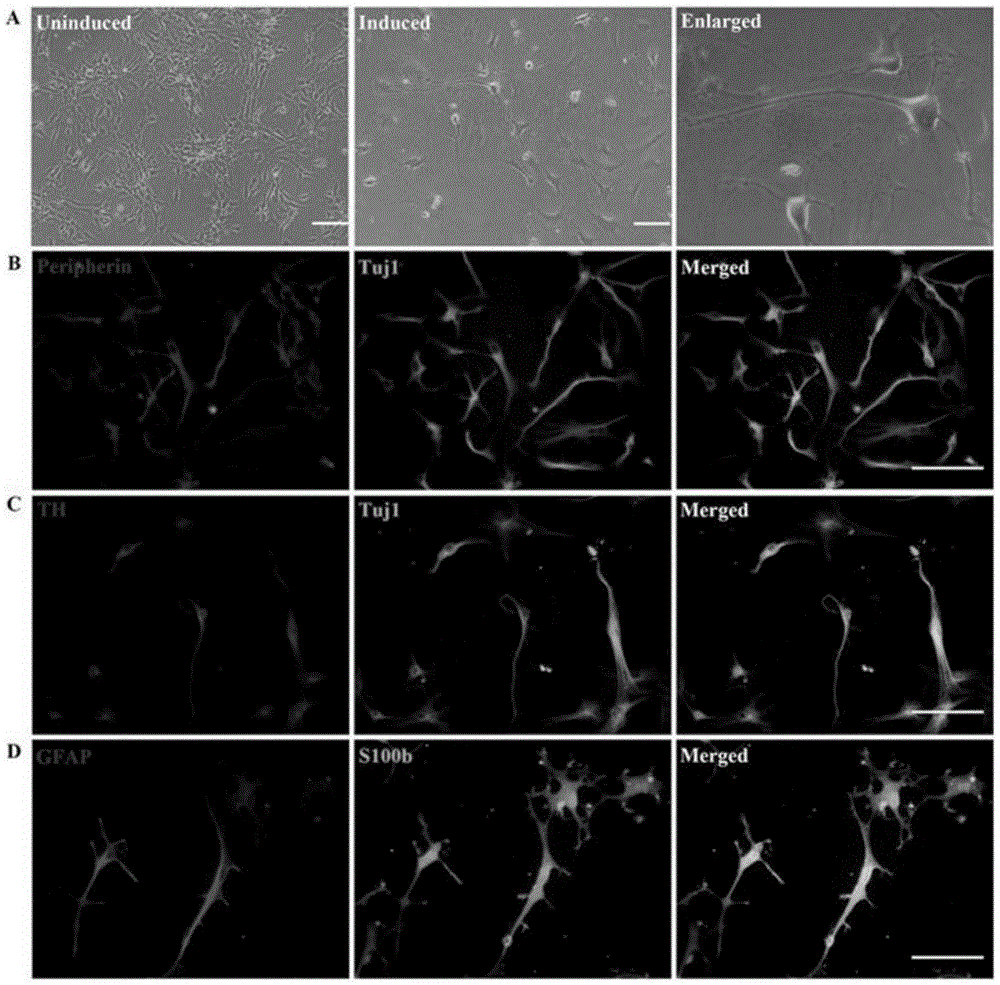
[0057] Expand the obtained hNCSCs cells, and replace them with Leydig cells (LCs) differentiation medium (DMEM-F12 medium, add 2% FCS, 10ngPDGF-BB, 1ng / mlLH, 1nM thyroid hormone (T3 ), 70ng / mlIGF-I), induced for 14 days, cell differentiation was carried out, cell supernatant was collected, and cells were fixed. The expressions of mature LCs-related markers (LCs markers 3β-HSD, P450c17, steroidogenic acute regulatory protein (StAR), and steroidogenic factor 1 (SF-1)) were detected by immunofluorescence, such as Figure 5 As shown, immunostaining analysis indicated that hNCSCs-induced differentiation cells (hNCSCs-LCs) expressed 3β-HSD, P450C17, StAR, SF-1. The testosterone level in the culture medium was measured by testosterone ELISA detection, such as Image 6 As shown, it was found that hNCSCs-LCs gradually increased testosterone secretion after differentiation in v...
Embodiment 3
[0058] Example 3 The effect of iPS-hNCSCs-LCs (or called hNCSCs-LCs) in vivo
[0059] Previous studies have shown that the specific apoptosis inducer of Leydig cells in rats treated with ethylene dimethanesulfonate (EDS) for 4 days can deplete Leydig cells, so the EDS model was established by intraperitoneal injection of EDS into rats. Three groups of adult rats were selected, which were normal control group, EDS-control group and cell transplantation group. The normal control group was intraperitoneally injected with the same volume of normal saline on the 0th day and the 4th day. EDS-control group rats were intraperitoneally injected with EDS (75 mg / kg body weight) on day 0, and injected 20 μl of normal saline (10 μl / unilateral testis) into testes on day 4. The rats in the cell group were intraperitoneally injected with EDS (75 mg / kg body weight) on day 0, and on day 4, hNCSCs-LCs (1.5×10 6 resuspended in 10 μl PBS / single testis) and transplanted into rat testes. Serum te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com