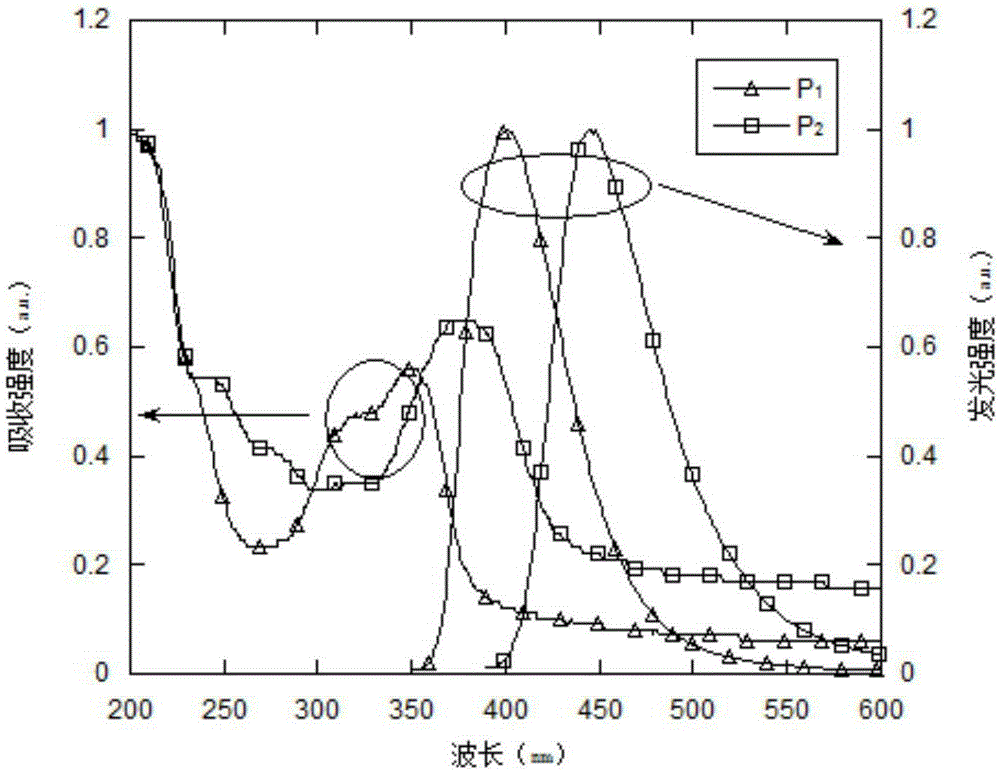
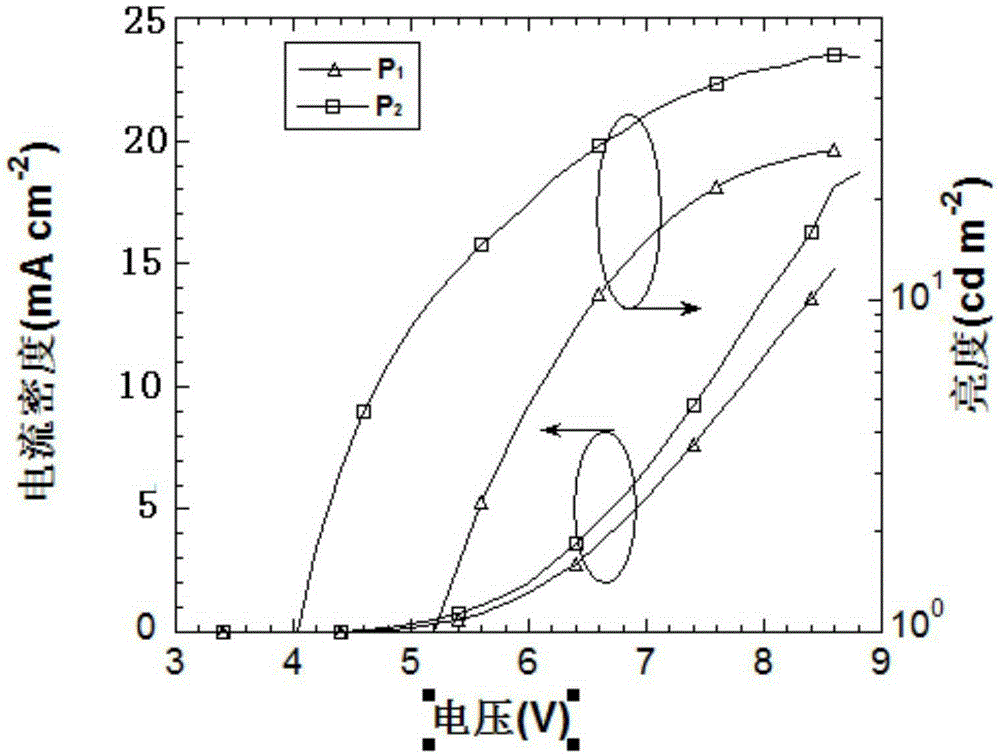
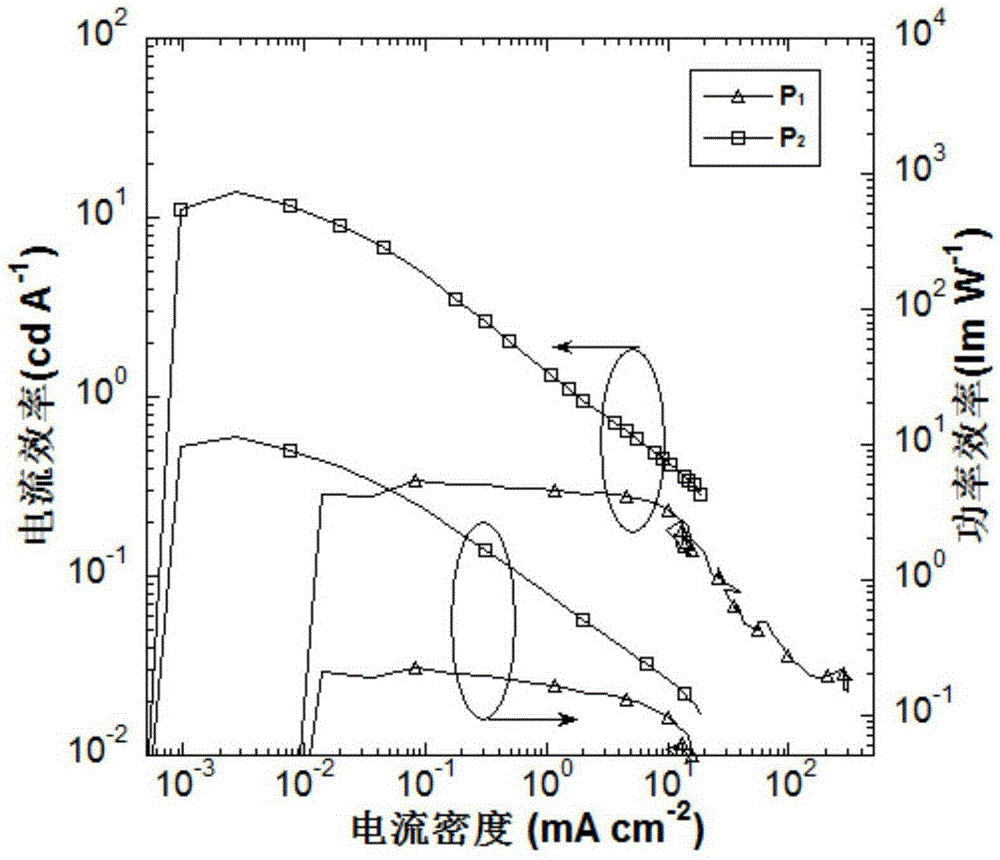
Organic small molecule luminescent material and organic electroluminescent device prepared from same
A technology of electroluminescent devices and luminescent materials, applied in luminescent materials, electric solid devices, electrical components, etc., can solve the problems of large CIE chromaticity coordinate y value, etc., and achieve good reproducibility, high device efficiency, and definite molecular weight Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment P 1 The preparation comprises the following preparation steps:
[0029] m 1 Synthesis of : Methyl 2-aminobenzoate (50mmol, 7.588g) was dissolved in 100ml of THF, cooled to 0°C, and methylmagnesium bromide (3.0MinTHF, 66.6mL, 200mmol) was added dropwise, and reacted for 24h . After the reaction was completed, THF was removed with a rotary evaporator, extracted with dichloromethane, and 6.19 g of a brown liquid was obtained after column separation, with a yield of 82%. 1 HNMR (500MHz, CDCl 3 , δ, ppm): 7.0-7.15 (m, 2H), 6.5-6.7 (m, 2H), 3.2-4.2 (s, 2H), 1.5-1.6 (s, 6H). The above reaction is shown in the following formula:
[0030]
[0031] m 2 Synthesis of: under the protection of argon, 4,4'-dibromodiphenyl sulfone (10mmol, 3.76g), M 1 (20mmol, 3.02g) were dropped into a 250ml three-necked reaction flask, and 100ml of toluene was added to dissolve them. After dissolving, sodium tert-butyl alkoxide (4.8g, 50mmol), tri-tert-butylphosphine (0.5ml...
Embodiment 2
[0036] This embodiment P 2 The preparation comprises the following preparation steps:
[0037] m 4 Synthesis of: under the protection of argon, 4,4'-dibromobenzophenone (10mmol, 3.4g), M 1(20mmol, 3.02g) were dropped into a 250ml three-necked reaction flask, and 100ml of toluene was added to dissolve them. After dissolving, sodium tert-butyl alkoxide (4.8g, 50mmol), tri-tert-butylphosphine (0.5ml, 1M / L) and palladium acetate (112mg, 0.5mmol) were added sequentially, and the system turned dark green. After feeding, heat to 110°C, reflux, and react for 24 hours. After the reaction was completed, the product was extracted with dichloromethane and washed with water, and 3.98 g of a yellow solid was obtained after column separation, with a yield of 83%. 1 HNMR (500MHz, DMSO, δ, ppm): 8.7-8.8 (s, 2H), 7.5-7.7 (d, 4H), 7.3-7.4 (d, 4H), 7.1-7.2 (d, 2H), 6.9-7.1 ( d, 2H), 5.2-5.3 (s, 2H), 1.5-1.6 (s, 12H).
[0038] m 5 The synthesis of: under the protection of argon, the M 4 (1...
Embodiment 3
[0042] This embodiment P 3 The preparation comprises the following preparation steps:
[0043] m 6 Synthesis of: under the protection of argon, 4-bromodiphenyl sulfone (10mmol, 2.97g), M 1 (20mmol, 3.02g) were dropped into a 250ml three-necked reaction flask, and 100ml of toluene was added to dissolve them. After dissolving, sodium tert-butyl alkoxide (4.8g, 50mmol), tri-tert-butylphosphine (0.5ml, 1M / L) and palladium acetate (112mg, 0.5mmol) were added sequentially, and the system turned dark green. After feeding, heat to 110°C, reflux, and react for 24 hours. After the reaction was completed, extracted with dichloromethane, washed with water, and separated by a column to obtain 3.19 g of a yellow solid with a yield of 87%. 1 HNMR (500MHz, DMSO, δ, ppm): 8.7-8.8 (s, 2H), 7.5-7.7 (d, 4H), 7.3-7.4 (d, 4H), 7.1-7.2 (d, 2H), 6.9-7.1 ( d, 2H), 5.2-5.3 (s, 2H), 1.5-1.6 (s, 12H).
[0044] m 7 The synthesis of: under the protection of argon, the M 6 (10mmol, 3.67g), 50ml of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com