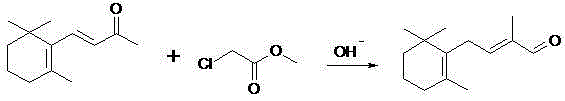
Synthetic method for vitamin A intermediate C14 aldehyde
A synthetic method and intermediate technology, which is applied in the field of synthesis of vitamin A intermediates, can solve the problems of difficult product purification and low yield of synthetic methods, and achieve the effects of high yield and purity, simple route, and avoid deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of Chloroisobutenyl Methyl Ether Grignard Reagent
[0032] Put 36.5 grams (1.1mol) of magnesium powder in a four-necked flask, add 300ml of tetrahydrofuran, stir vigorously, the temperature rises to 66°C, and add 1 particle of iodine activated magnesium powder, and weigh 132.5 grams (1.1mol) of chlorinated Dissolve isobutenyl methyl ether in 300ml of tetrahydrofuran, slowly drop it into the flask, observe the boiling of tetrahydrofuran, and see that the initiation is successful when a large amount of foam is seen in the flask, then lower the temperature of the water bath to 40°C to cool down the reaction system, and keep the flask The internal reaction was carried out in a slightly boiling state. After the chloroisobutenyl methyl ether was dripped, the temperature was raised to 50° C. and the temperature was continued for 2.0 hours.
[0033] 2. Preparation of C14 aldehyde
[0034] Cool the prepared chloroisobutenyl methyl ether Grignard reagent to -15°C,...
Embodiment 2
[0036] In this embodiment, the acid used is hydrochloric acid, the reaction solvent is methyl tetrahydrofuran, the condensation temperature is from -20°C to 50°C, and the condensation holding time is 1.0-10.0 hours. All the other are identical with embodiment 1.
Embodiment 3
[0038] In this embodiment, the acid used is acetic acid, the reaction solvent is diethyl ether, the condensation temperature is from -20°C to 50°C, and the condensation holding time is 1.0-10.0 hours. All the other are identical with embodiment 1.
[0039] As can be seen from the above examples, a method for synthesizing a vitamin A intermediate C14 aldehyde of the present invention has a simple route, does not need to pass through a β-ionone intermediate, and is directly condensed from cyclic citral, and the yield and purity of the product are high; The product is obtained in an acidic system, which avoids the deterioration of the product in a high temperature and strong alkali environment; the condensate of the chloroisobutenyl methyl ether Grignard reagent is hydrolyzed into olefins and alkene ethers, and the hydrolysis is carried out to obtain aldehydes in one step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com