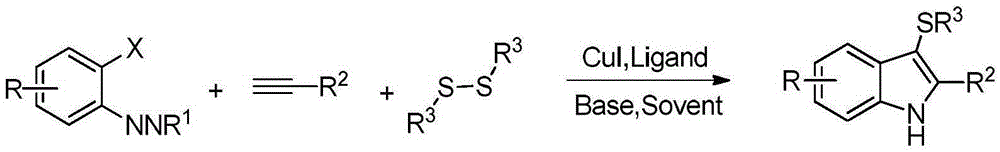Preparation method for polysubstituted indole derivatives
A technology of indole derivatives and multi-substitution, which is applied in the field of preparation of multi-substituted indole derivatives, can solve problems such as pollution and complex preparation methods, and achieve the effect of strong operability and simple technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
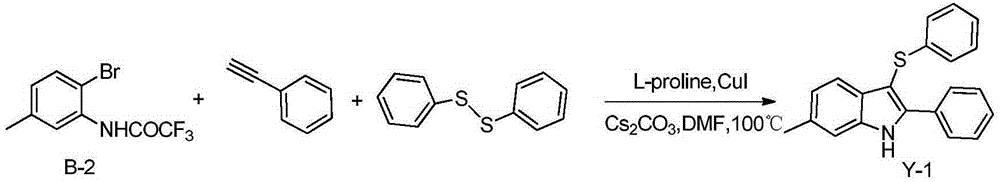
Embodiment 16
[0016] Example 16-Methyl-2-phenyl-3-(phenylthio)-1H-indole
[0017]
[0018] In a 25mL three-neck flask, add B-2 (282mg, 1mmol), and then add Cs 2 CO 3 (652mg, 2mmol), diphenyl disulfide (109mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous iodide (19mg, 0.1mmol), phenylacetylene (102mg, 1mmol) , 5mLDMF, under the protection of nitrogen, react in an oil bath at 100°C for 24h, add 5mL water after cooling, extract with 5mL ethyl acetate each time, after repeating four times, the extract is washed with saturated brine, anhydrous sulfuric acid After drying over sodium, the filtered liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 274.4 mg of a light yellow solid with a yield of 87%.
[0019] 1 HNMR (500MHz, CDCl 3 )δ8.48(s,1H),7.78(d,J=7.4Hz,2H),7.57(d,J=8.0Hz,1H),7.46(t,J=7.4Hz,2H),7.41(t, J=7.1Hz, 1H), 7.27(s, 1H), 7.22(t, J=7.6Hz, 2H), 7.17(d, J=7.4Hz, 2H)...
Embodiment 26
[0020] Example 26-Nitro-2-phenyl-3-(phenylthio)-1H-indole
[0021]
[0022] In a 25mL three-neck flask, add B-3 (313mg, 1mmol), and then add Cs 2 CO 3 (652mg, 2mmol), diphenyl disulfide (109mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous iodide (19mg, 0.1mmol), phenylacetylene (102mg, 1mmol) , 5mLDMF, under the protection of nitrogen, react in an oil bath at 100°C for 24h, add 5mL water after cooling, extract with 5mL ethyl acetate each time, after repeating four times, the extract is washed with saturated brine, anhydrous sulfuric acid After drying over sodium, the filtered liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 294.4 mg of a yellow solid with a yield of 85%.
[0023] 1 HNMR (500MHz, CDCl 3 )δ8.54(s,1H),7.80(dd,J=7.1,1.2Hz,2H),7.62(dd,J=7.7,5.0Hz,1H),7.51-7.39(m,4H),7.26-7.19 (m,4H),7.17-7.07(m,2H).
Embodiment 3
[0024] Example 32-((2-phenyl-1H-indol-3-yl)thio)benzo[d]thiazole
[0025]
[0026] In a 25mL three-neck flask, add B-1 (268mg, 1mmol), and then add Cs 2 CO 3 (652mg, 2mmol), benzothiazole disulfide (166mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous iodide (19mg, 0.1mmol), phenylacetylene (102mg, 1mmol), 5mL of DMF, under the protection of nitrogen, react in an oil bath at 100°C for 24h, add 5mL of water after cooling, extract with 5mL of ethyl acetate each time, after repeating four times, the extract is washed with saturated saline, anhydrous sodium sulfate After drying and filtering, the liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 272.4 mg of a light yellow solid with a yield of 76%.
[0027] 1 HNMR (500MHz, CDCl 3 )δ10.63(s,1H),7.81(s,1H),7.55(s,1H),7.47(s,3H),7.39(s,2H),7.30(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com