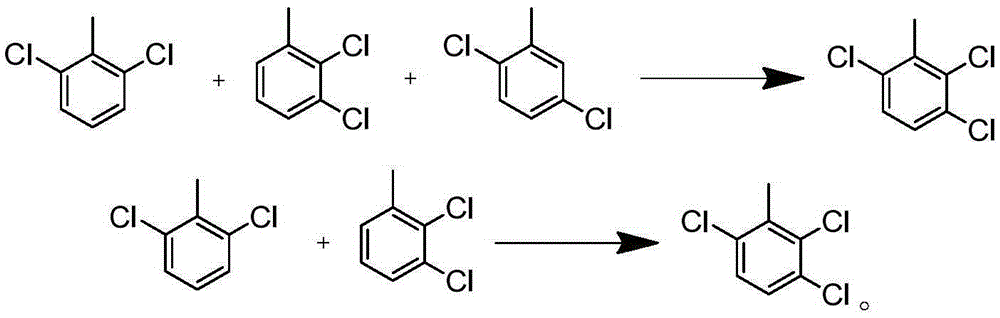
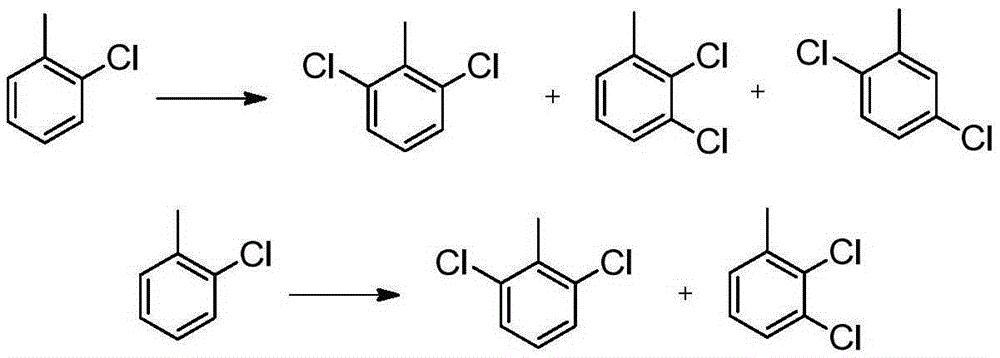
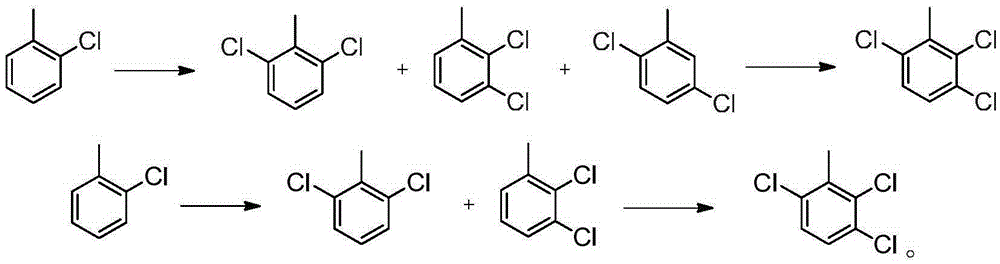
Synthesizing method for 2,3,6-trichlorobenzoic acid and intermediate of 2,3,6-trichlorobenzoic acid
A technology of trichlorotoluene and dichlorotoluene, applied in 2 fields, can solve the problems of complex preparation methods, high production costs, poor reaction selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation method of embodiment 1M-ZSM-12
[0065] The preparation method of M-ZSM-12 is: take a certain amount of ZSM-12 molecular sieve and place it in a round-bottomed flask, add 5 times of mass equivalents of hydrochloric acid with a mass concentration of 5% (the mass concentration refers to the mass of hydrogen chloride) % of the total mass of hydrochloric acid) and one-third of the mass equivalent of metal chloride, reflux and stir at 100°C for 20 hours, wash with water until neutral, then dry at 120°C for 24 hours, and activate at 400°C for 5 hours . When the metal chloride is zinc dichloride, ferric chloride, antimony trichloride or titanium tetrachloride, the obtained composites are respectively recorded as Zn-ZSM-12, Fe-ZSM-12, Sb-ZSM-12, Ti - ZSM-12.
Embodiment 2 2
[0066] The preparation of embodiment 2 dichlorobenzene mixtures
[0067] Add 100g of o-chlorotoluene and 1g of Fe-ZSM-12 into a four-necked round-bottomed flask, stir at 25°C for 30 minutes, and then feed 49.8g of chlorine gas under normal pressure at a rate of 10g / h. After reacting for about 5 hours, the conversion rate is detected by gas phase. 87%, stop logical chlorine. Obtained 124.2 g of reaction liquid, composed of o-chlorotoluene 13%, 2,6-dichlorotoluene 42%, 2,3-dichlorotoluene 27%, 2,5-dichlorotoluene 16%, trichlorotoluene and other impurities 2.0%. Rectification, distilled o-chlorotoluene was recovered and used mechanically, and the dichlorobenzene mixture was 112.3g, with a yield of 88.3% (95.7% after applying mechanically), which could be directly used in the next reaction.
Embodiment 3 2
[0068] The preparation of embodiment 3 dichlorobenzene mixtures
[0069]Add 100g of o-chlorotoluene and 1g of Sb-ZSM-12 into a four-necked round-bottomed flask, stir at 25°C for 30 minutes, and then feed 48.2g of chlorine gas under normal pressure at a rate of 10g / h. After reacting for about 5 hours, the conversion rate is detected by gas phase. 85%, stop passing chlorine. Obtained 123.4 g of reaction solution, composed of 15% o-chlorotoluene, 55% 2,6-dichlorotoluene, 23% 2,3-dichlorotoluene, 6% 2,5-dichlorotoluene, trichlorotoluene and other impurities 1.0%. The ortho-chlorotoluene was distilled by rectification and recovered and used mechanically. The dichlorobenzene mixture distilled was 109.5g, with a yield of 86.1% (96.8% after applying mechanically), which could be directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com