Preparation method for copper selenide film
A technology of copper selenide and thin film, which is applied in the direction of electrolytic inorganic material coating, etc., can solve the problems of forming solar cells, and achieve the effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take CuCl 2 0.34g (2.5mM), KCL7.45g (0.1M), SeO 2 Dissolve 0.50g (4.5mM) in one liter of distilled water in turn, use hydrochloric acid to titrate, and keep the pH value of the solution at 1.5 to obtain the electrolyte; add the electrolyte to the electrolytic cell; the ITO glass substrate is used as the working electrode, and the Pt is used as the working electrode. For the counter electrode, a saturated calomel electrode (SCE) was used as a reference electrode, and a water bath was used to maintain the temperature of the reaction system at 90°C. A voltage of -0.10V relative to the reference electrode was applied to the working electrode through an electrochemical workstation, and the reaction time was 30 minutes. A black product is obtained on the working electrode, which is the product copper selenide flake film.
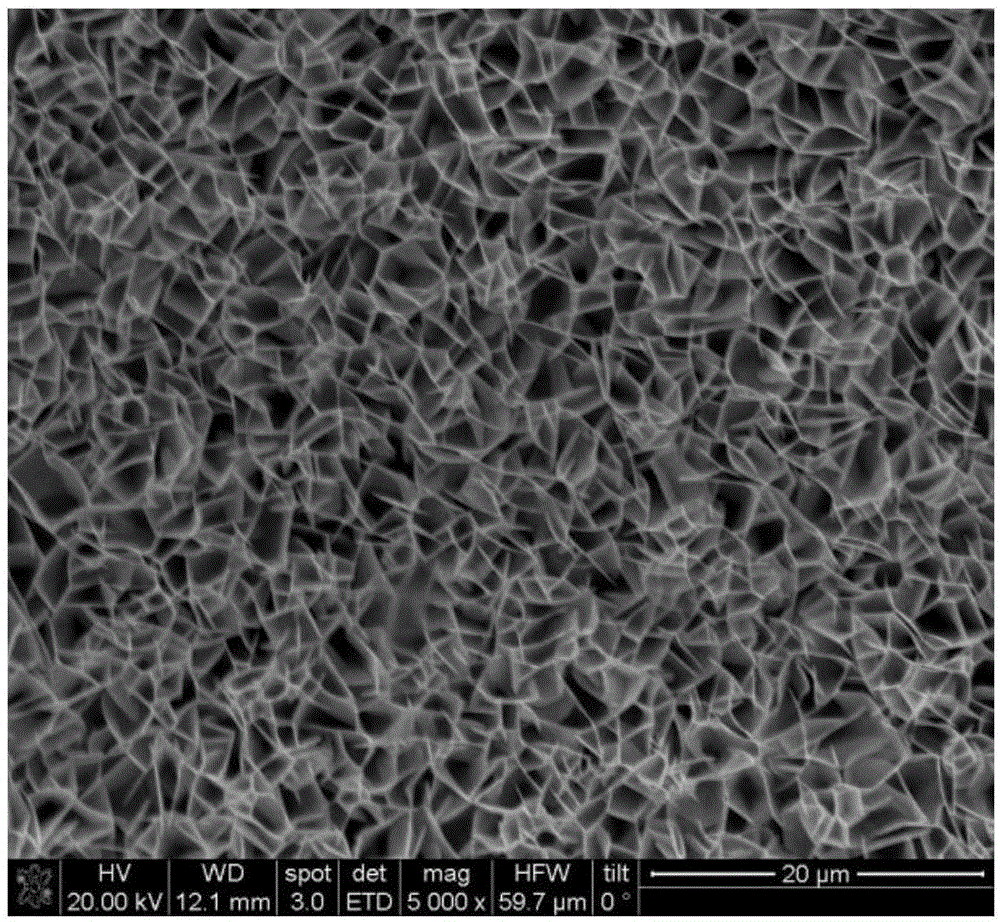
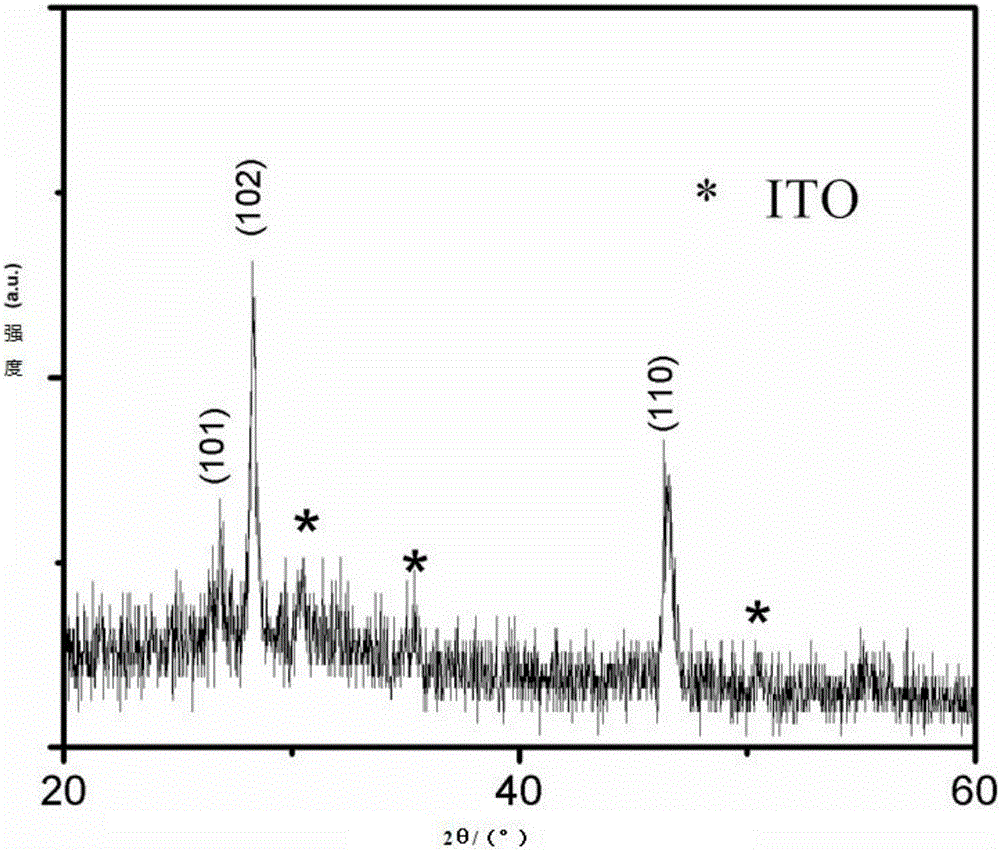
[0025] product appearance see figure 1 The SEM photo, the phase structure see figure 2 The XRD spectrum.
Embodiment 2
[0027] Take CuCl 2 0.34g (2.5mM), KCL7.45g (0.1M), SeO 2 Dissolve 0.50g (4.5mM) in one liter of distilled water in turn, use hydrochloric acid to titrate, and keep the pH value of the solution at 1.5 to obtain the electrolyte; add this electrolyte to the electrolytic cell; FTO glass substrate as the working electrode, Pt as the For the counter electrode, a saturated calomel electrode (SCE) was used as a reference electrode, and a water bath was used to maintain the temperature of the reaction system at 20°C. A voltage of -0.10V relative to the reference electrode was applied to the working electrode through an electrochemical workstation, and the reaction time was 30 minutes. A black product is obtained on the working electrode, which is the product copper selenide flake film.
[0028] product appearance see figure 2 SEM photographs.
Embodiment 3
[0030] Take CuCl 2 0.34g (2.5mM), KCL7.45g (0.1M), SeO 2 Dissolve 0.50g (4.5mM) in one liter of distilled water in turn, use hydrochloric acid to titrate, and keep the pH value of the solution at 1.5 to obtain the electrolyte; add the electrolyte to the electrolytic cell; ATO glass substrate as the working electrode, Pt as the For the counter electrode, a saturated calomel electrode (SCE) was used as a reference electrode, and a water bath was used to maintain the temperature of the reaction system at 90°C. A voltage of -0.15V relative to the reference electrode was applied to the working electrode through an electrochemical workstation, and the reaction time was 30 minutes. A black product is obtained on the working electrode, which is the product copper selenide flake film.
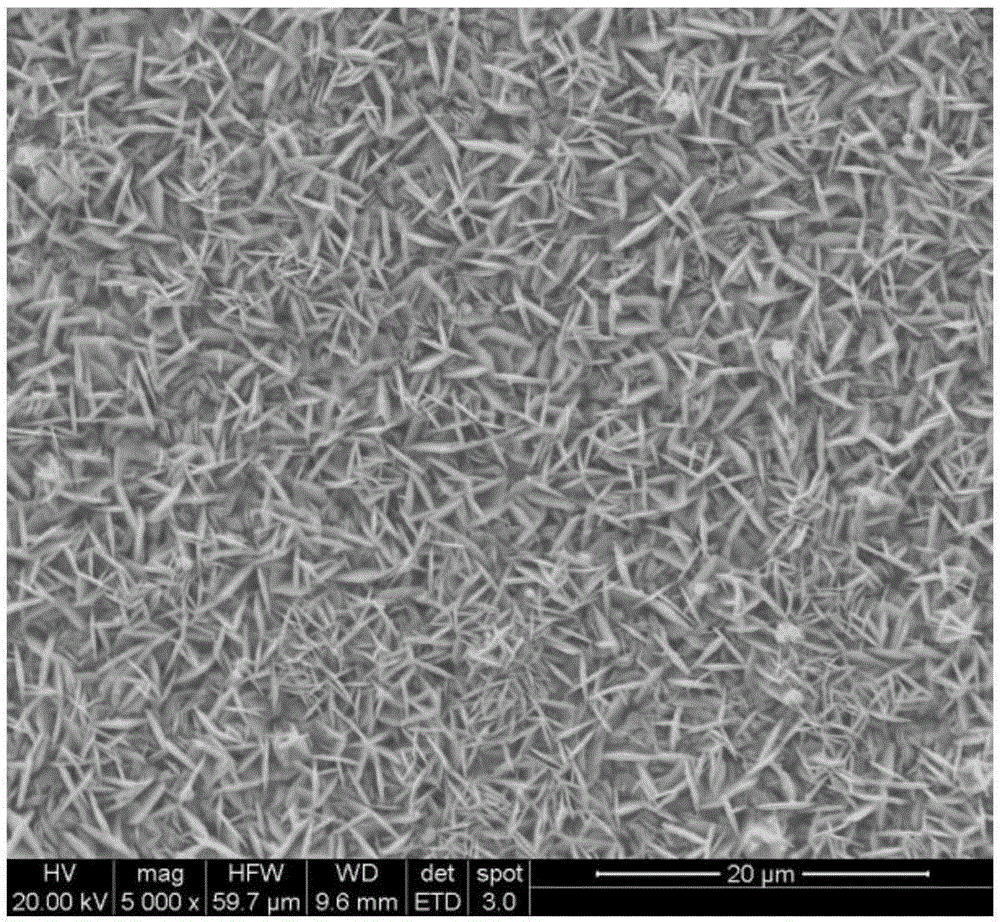
[0031] product appearance see image 3 SEM photographs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com