Compound, preparation method thereof, oil absorbing resin and synthesis method thereof
A synthesis method and technology of oil-absorbing resins, applied in the field of oil-absorbing resins, can solve the problems of low resin strength, reduced resin practicability, and inability to meet the requirements of the desorption process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
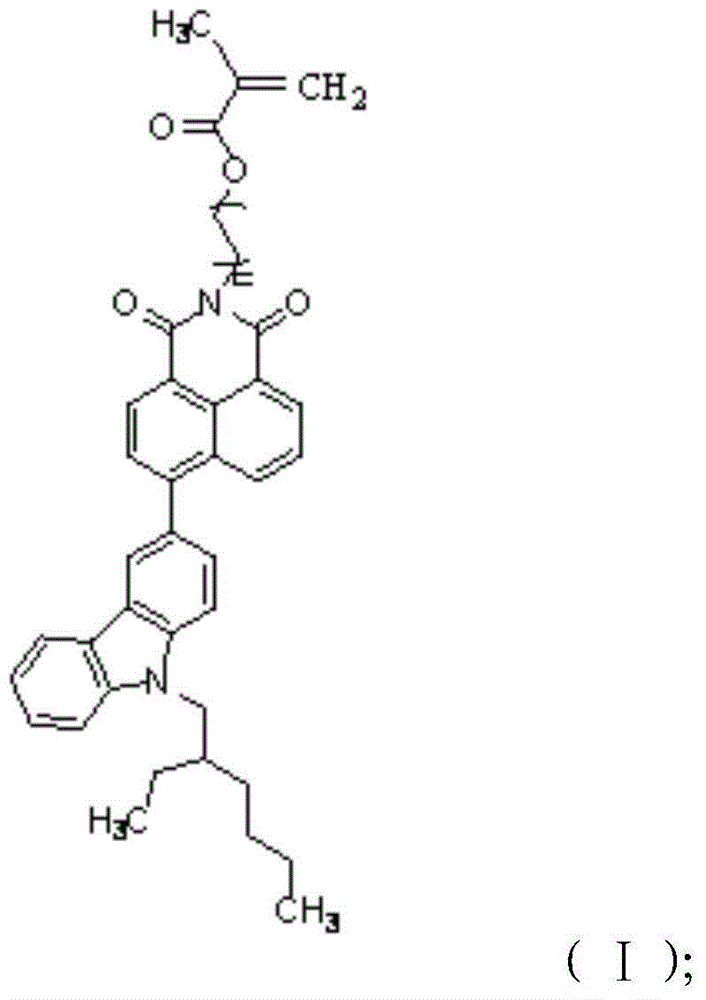
[0030] The present application also provides a preparation method of the compound having the structure of formula (I), including:
[0031] 6-(9-(2-ethylhexyl)-6-nitro-9H-carbazol-3-olyl)-2-(6-hydroxyethyl)-1H-benzisoquinoline-1, 3(2H)-diketone is reacted with methacryloyl chloride to obtain a compound having the structure of formula (I).
[0032] In the above process, the 6-(9-(2-ethylhexyl)-6-nitro-9H-carbazol-3-alcohol base)-2-(6-hydroxyethyl)-1H-benzoiso The substitution reaction between quinoline-1,3(2H)-dione and the methacryloyl chloride occurs. During the reaction, the 6-(9-(2-ethylhexyl)-6-nitro-9H-carbazole-3-alcohol base)-2-(6-hydroxyethyl)-1H-benzene And isoquinoline-1,3 (2H)-diketone is first dissolved in tetrahydrofuran and triethylamine, and described methacryloyl chloride is dissolved in tetrahydrofuran, and then the two are reacted, and obtained has formula (Ⅰ ) structure compounds. The 6-(9-(2-ethylhexyl)-6-nitro-9H-carbazol-3-alcohol base)-2-(6-hydroxyeth...
Embodiment 1
[0050] Preparation of 9-(2-ethylhexyl)-9H-carbazole: carbazole (4.0 g, 23.9 mmol), N,N-methylformamide (DMF, 30 mL), potassium hydroxide powder (2.53 g, 0.5 mol), added in a 200 ml round bottom flask, stirred for 30 min; 1-bromo-2-ethylhexyl (4.86 g, 25.19 mmol) was dissolved in 10 ml of DMF and dropped into the above mixed solution, stirred at room temperature for 12 h The reaction solution was poured into 400 milliliters of deionized water, dried over magnesium sulfate after being extracted with dichloromethane, and separated with a silica gel column (dichloromethane and sherwood oil 25:1 volume ratio) after dichloromethane was evaporated to get product 5.16g, 77% yield). 1HNMR (400MHz, CDCl 3 )δ8.11(d, J=7.7Hz, 2H), 7.52-7.45(m, 2H), 7.40(d, J=7.3Hz, 2H), 7.30~7.20(m, 2H), 4.23-4.13(m , 2H), 2.16-2.02(m, 1H), 1.47-1.22(m, 8H), 0.92(t, J=7.5Hz, 3H), 0.87(t, J=7.2Hz, 3H).
[0051] Preparation of 3-bromo-9-(2-ethylhexyl)-9H-carbazole: Add product 1 (5.16g, 18.47mmol) and 50...
Embodiment 2
[0058] Add water and 1g of dispersant polyvinyl alcohol into the reaction bottle, stir at 40°C to cool the polyvinyl alcohol solvent, add 90 g of long-chain methacrylate monomers and 10 g of monomers synthesized in Example 1, 0.3 ~1.0g initiator azobisisobutylcyanide and 0.3~1.0g crosslinking agent divinylbenzene, react for several hours under nitrogen protection, cool and filter, wash with 60~80℃ water and dry to obtain 85g Oil-absorbing resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com