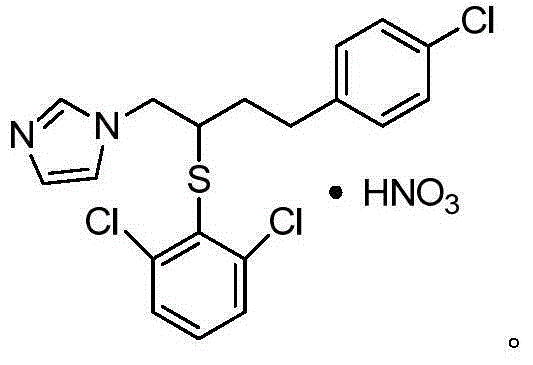
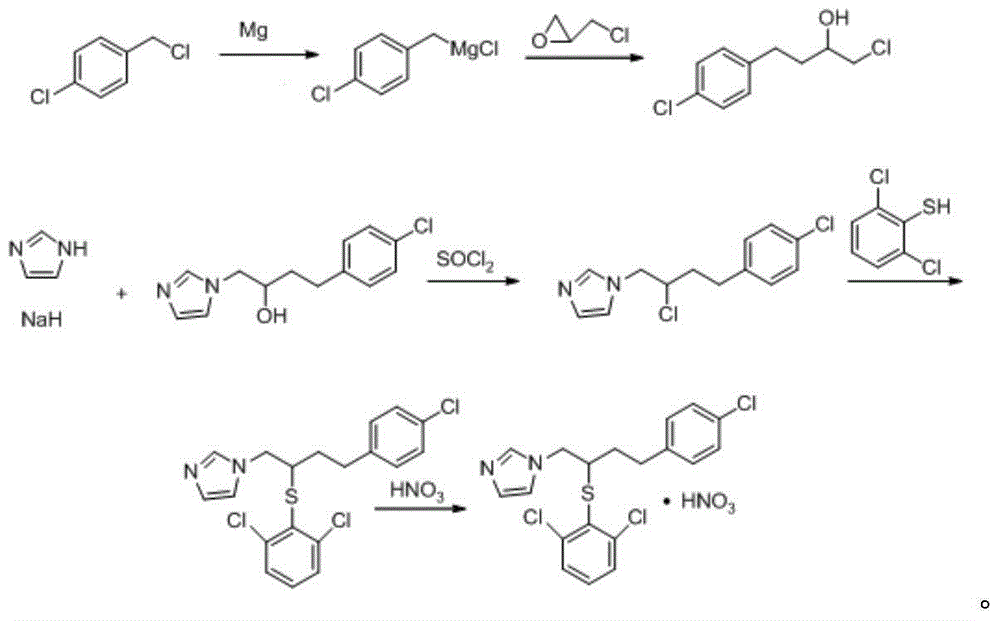
Method for industrially producing butoconazole nitrate intermediate
A technology for butconazole nitrate and intermediates, which is applied in the field of industrial production of butconazole nitrate intermediates, can solve the problems of high cost, complicated production process of butconazole nitrate, product yield and purity that cannot meet industrialized production, and the like. Easy to operate, suitable for large-scale industrial production, reasonable in raw materials and dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
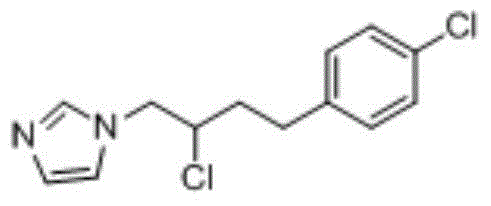
[0051] 1-(2-Chloro-4-(4-chlorophenyl)butyl)-1hydro-imidazole was prepared according to the following procedure:
[0052] (1) Take 3kg of 1-(2-hydroxy-4-(4-chlorophenyl)butyl)-1hydro-imidazole, fully dissolve it in 30kg of dichloromethane, and add it dropwise at a rate of 1.5ml / s at 20°C 3.5 kg of thionyl chloride, slowly heated up to 33°C, held for 1 hour, then refluxed at 60°C for 1 hour, slowly cooled to below 15°C to obtain a reaction solution;
[0053] (2) Add water at 10°C at a rate of 0.5ml / s to the reaction solution obtained in step (1) while stirring, the volume ratio of the reaction solution to water is 0.005:1, and then add water at a rate of 1.0 to 1.5kg / L Add anhydrous sodium carbonate, filter with suction, discard the solid, concentrate the liquid at 55°C until no dichloromethane evaporates, and dry to obtain the obtained product.
[0054] After testing, the yield of the product in this example was 99.18%. The target product and impurity content were detected by...
Embodiment 2
[0056] Compared with Example 1, the difference is only that the step (1) is specifically:
[0057] (1) Take 2kg of 1-(2-hydroxy-4-(4-chlorophenyl)butyl)-1hydro-imidazole, fully dissolve it in 25kg of dichloromethane, and add 2kg of it dropwise at a rate of 1ml / s at 15°C For thionyl chloride, slowly raise the temperature to 30°C, keep it warm for 0.5 hours, then reflux at 55°C for 0.5 hours, and slowly cool to 10°C to obtain a reaction solution.
[0058] After testing, the yield of the product in this example was 97.32%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target product 1-(2-chloro-4-(4-chlorophenyl)butyl)-1 hydrogen-imidazole is 98.93%, 1-(1-chloro-4-(4-chlorophenyl The content of )butane-2-yl)-1 hydrogen-imidazole is 0.57%, and the content of 1-(2-hydroxy-4-(4-chlorophenyl)butyl)-1 hydrogen-imidazole is 0.25%.
Embodiment 3
[0060] Compared with Example 1, the difference is only that the step (1) is specifically:
[0061] (1) Take 5 kg of 1-(2-hydroxy-4-(4-chlorophenyl) butyl)-1 hydrogen-imidazole, fully dissolve it in 35 kg of dichloromethane, and add 5 kg of it dropwise at a rate of 2 ml / s at 25°C Thionyl chloride was slowly heated to 35°C, kept for 1.5 hours, then refluxed at 65°C for 1.5 hours, and slowly cooled to 20°C to obtain a reaction solution.
[0062] After testing, the yield of the product of this example was 98.14%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target product 1-(2-chloro-4-(4-chlorophenyl)butyl)-1 hydrogen-imidazole is 98.97%, 1-(1-chloro-4-(4-chlorophenyl )butane-2-yl)-1H-imidazole content was 0.58%, and 1-(2-hydroxy-4-(4-chlorophenyl)butyl)-1H-imidazole content was 0.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com