Fasudil hydrochloride injection and preparing method thereof
A technology of fasudil hydrochloride and injection, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of complicated preparation process, inconvenient industrial production, and high cost. Achieve the effect of simple preparation process, stable quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
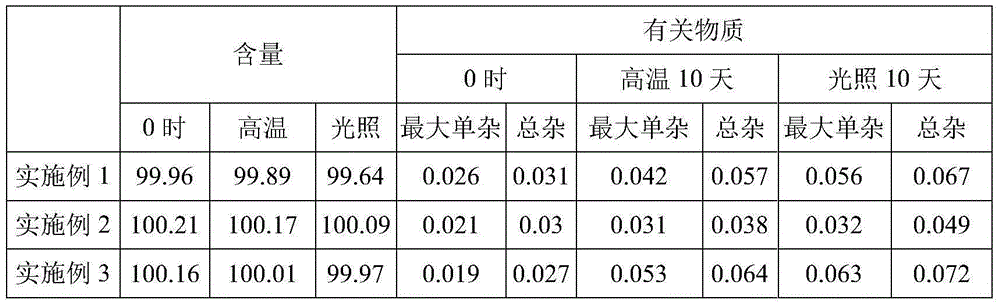
Embodiment 1
[0015] Raw materials and consumables: Fasudil hydrochloride 30g, sodium citrate 10g, disodium edetate 0.01g, activated carbon for needles 0.5g, water for injection to 2000ml, made into 1000 sticks.
[0016] Preparation method: 1) Measure 80% of the total amount of water for injection, put into the prescribed amount of fasudil hydrochloride, sodium citrate, and disodium edetate, and stir to make them completely dissolve; 2) adjust with 1mol / L NaOH solution pH to 5.81; 3) Add the prescribed amount of activated carbon for needles, stir and absorb for 20 minutes, decarbonize and filter; 4) Add water for injection to the full amount, stir for 10 minutes, mix well and fine filter with a 0.22 μm filter; 5) Filter the filtrate Fill in 2ml brown ampoules and seal; 6) Sterilize the sealed ampoules at 121°C for 15 minutes, inspect the sterilized ampoules with light, and pack.
Embodiment 2
[0018] Raw materials and consumables: Fasudil hydrochloride 30g, sodium citrate 10g, edetate disodium 0.01g, dihydroconiferyl alcohol γ-O-α-L-rhamnoside 0.02g, activated carbon for needles 0.5g , Water for injection to 2000ml, made into 1000 sticks.
[0019] Preparation method: 1) Measure 80% of the total amount of water for injection, and drop into prescribed amounts of fasudil hydrochloride, sodium citrate, disodium edetate, dihydroconiferyl alcohol γ-O-α-L-ratamine 2) Use 1mol / L NaOH solution to adjust the pH to 5.81; 3) Add the prescribed amount of activated carbon for needles, stir and adsorb for 20 minutes, and decarbonize and filter; 4) Supplement water for injection to the full amount, stir for 10 5) Fill the filtrate into a 2ml brown ampoule and seal it; 6) Sterilize the sealed ampoule at 121°C for 15 minutes, inspect the sterilized ampoule with light, Package.
Embodiment 3
[0021] Raw materials and consumables: Fasudil hydrochloride 30g, sodium citrate 10g, disodium edetate 0.025g, activated carbon for needles 0.5g, water for injection to 2000ml, made into 1000 sticks.
[0022] Preparation method: 1) Measure 80% of the total amount of water for injection, put into the prescribed amount of fasudil hydrochloride, sodium citrate, and disodium edetate, and stir to make them completely dissolve; 2) adjust with 1mol / L NaOH solution pH to 5.99; 3) Add the prescribed amount of activated carbon for needles, stir and absorb for 20 minutes, decarbonize and filter; 4) Add water for injection to the full amount, stir for 10 minutes, mix well and fine filter with a 0.22 μm filter; 5) Filter the filtrate Fill in 2ml brown ampoules and seal; 6) Sterilize the sealed ampoules at 121°C for 15 minutes, inspect the sterilized ampoules with light, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com