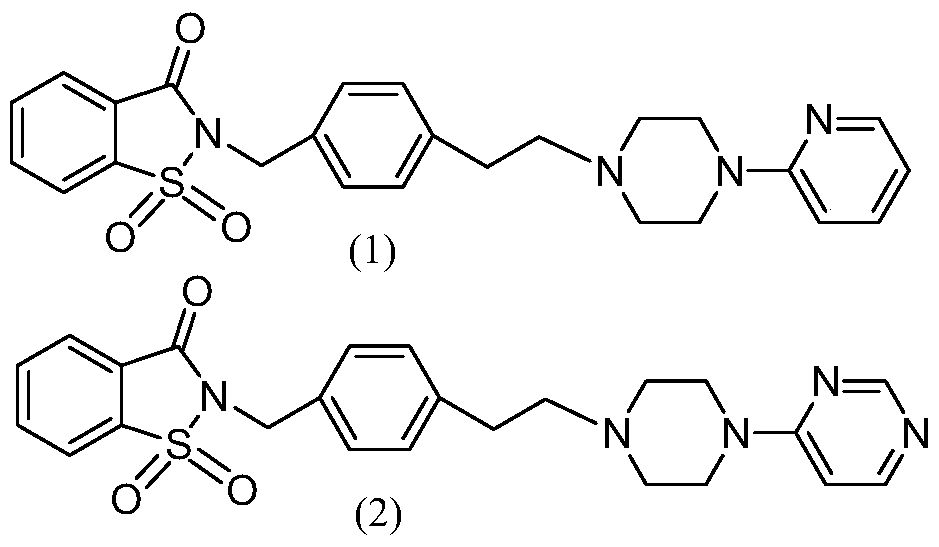
Arylpiperazine derivative ii and its salt, preparation method and use
A technology of arylpiperazine and derivatives, which is applied in the discovery of drug lead compounds and the field of medicinal chemistry, can solve the problems of inability to cure prostate cancer, inability to improve treatment results or survival rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
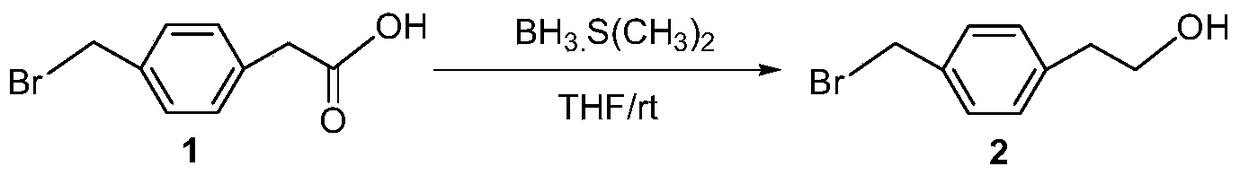
[0037] Embodiment 1: the preparation of intermediate 2
[0038]
[0039] Add 5g (0.021mol) 4-(bromoethane) phenylacetic acid, 100mL tetrahydrofuran into a 250mL round bottom flask, slowly add 21.9mL borane dimethyl sulfide complex (BMS, 2M in THF) at 0°C . The reaction mixture was reacted at 0° C. for 1 h, and then gradually returned to normal temperature. After the reaction was completed, water was slowly added to terminate the reaction, extracted with ethyl acetate (100 mL×3), the organic phases were combined, washed with water and saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated. The crude product was directly used in the next reaction without purification.
Embodiment 2
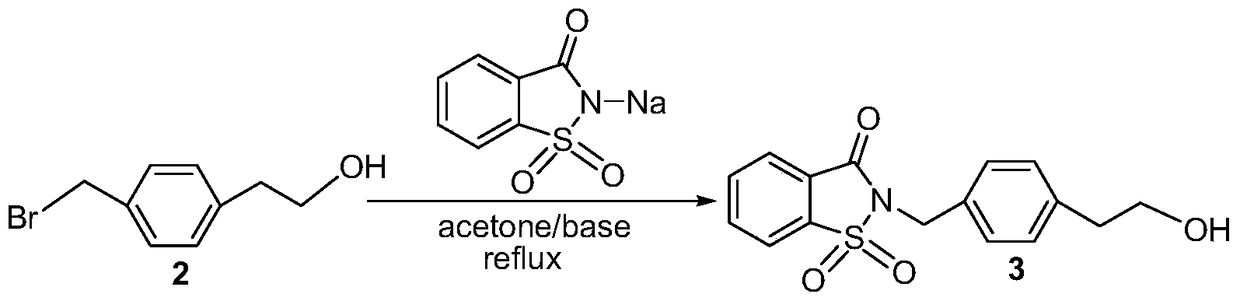
[0040] Embodiment 2: the preparation of intermediate 3
[0041]
[0042] Add 4g (18.7mmol) of intermediate 2, 3.85g (18.7mmol) of sodium saccharin, 10.32g (74.8mmol) of potassium carbonate, and 150mL of acetone into a 250mL round bottom flask, and react at 60°C for 16h. TLC showed the starting material was completely reacted. The reaction was stopped, filtered and concentrated. The crude product was purified by silica gel column chromatography, eluent: V (ethyl acetate): V (petroleum ether) = 1:6, to obtain 5.90 g of white solid, yield: 85% (using raw material 4-(bromoethane ) phenylacetic acid calculation). M.p.:97-98℃; MS(ESI,m / z):318.07[M+H] + ; 1 H NMR (500MHz, DMSO-d 6 )δin ppm: 8.33 (d, J = 7.7Hz, 1H, Ar-H), 8.15–7.98 (m, 3H, Ar-H), 7.33 (d, J = 8.0Hz, 2H, Ar-H), 7.20 (d, J=8.0Hz, 2H, Ar-H), 4.87(s, 2H, CH 2 ), 3.58 (td, J=7.0, 5.4Hz, 2H, CH 2 ),2.70(t,J=7.0Hz,2H,CH 2 ),2.23(t,J=5.2Hz,1H,OH); 13 C NMR (126MHz, DMSO-d 6 )δin ppm: 158.58, 139.19, 136.82, 135....
Embodiment 3
[0043] Embodiment 3: the preparation of intermediate 4
[0044]
[0045] In a 250 mL round bottom flask, add 4 g (12.62 mmol) of intermediate 3, 5.09 g (50.48 mmol) of triethylamine, 0.15 g of 4-(N,N-dimethyl)aminopyridine (catalytic amount), 100 mL of dichloromethane , a dichloromethane solution of 3.59 g (18.93 mmol) p-toluenesulfonyl chloride (TsCl) was slowly added at 0°C. The reaction mixture was reacted at 0° C. for 16 h, and TLC showed that the starting material was completely reacted. Slowly add water to terminate the reaction, extract with dichloromethane (100 mL×3), combine the organic phases, wash with water and saturated brine, dry over anhydrous magnesium sulfate, filter, and concentrate. The crude product was purified by silica gel column chromatography, eluent: V (ethyl acetate): V (petroleum ether) = 1:8, to obtain 4.75 g of white solid, yield: 80%. M.p.:119-120℃; MS(ESI,m / z):472.08[M+H] + ; 1 H NMR (400MHz, CDCl 3 )δin ppm: 8.33 (d, J = 7.7Hz, 1H, Ar-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com