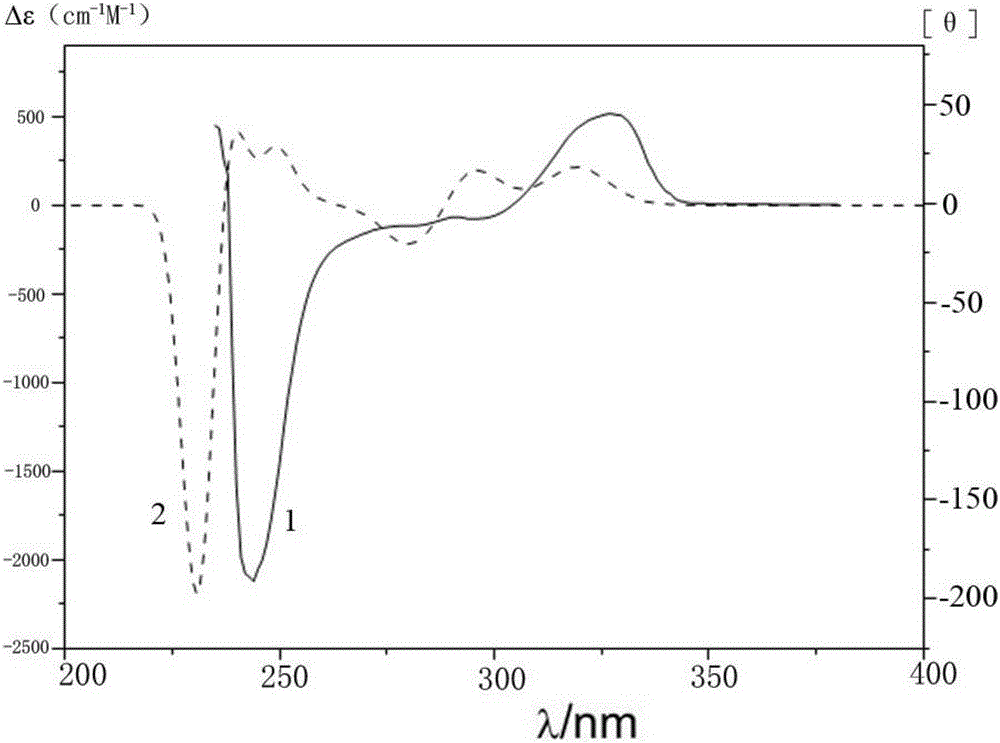
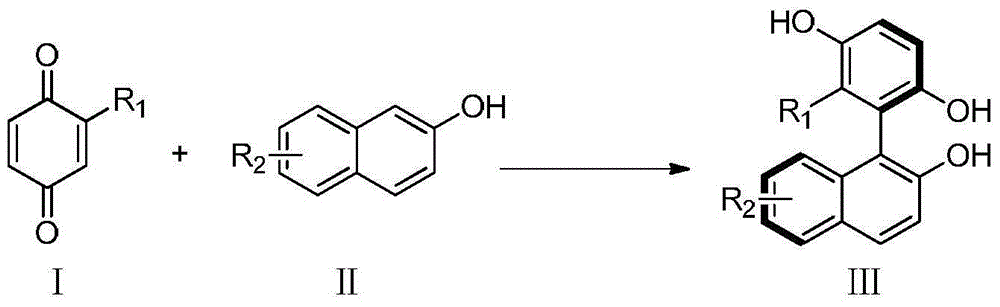
Method for catalyzing asymmetrically synthesized axially chiral biaryl diphenol
A synthetic method and asymmetric technology, applied in the field of catalytic asymmetric synthesis of axial chiral biaryl diphenols, can solve the problems of low yield, less research and reports, etc., and achieve the advantages of convenient source, easy preparation, and no heavy metal pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] General synthetic method of 1,4-dihydroxybenzene-2-carboxylate
[0035]
[0036] Dissolve 2,5-dihydroxybenzoic acid (6.3 g, 40 mmol) and substituted benzyl bromide (174 mmol) in DMF (N, N-dimethylformamide, 100 mL), and add KHCO under stirring 3 (10.7g, 107mmol) solid, the resulting mixture was heated to 70°C and stirred for 24h, then the reaction solution was cooled to room temperature, diluted with 1M dilute hydrochloric acid (200mL), extracted with DCM (100mLx2), and the obtained organic phase was washed with water (100mLx2), MgSO4 was dried, the solvent was concentrated, and DCM / PE (petroleum ether) = 2 / 1 was used as the eluting solvent, and the obtained residue was purified by silica gel column chromatography to obtain the product.
[0037]
[0038] Yield 74%; 1 HNMR (400MHz, CDCl 3 )δ10.32(s,1H),7.44-7.30(m,6H),7.02-6.99(m,1H),6.88(d,J=8.8Hz,1H),5.36(s,2H),4.70(s ,1H); 13 CNMR (100Hz, CDCl 3 ) δ 169.43, 155.86, 148.14, 135.21, 128.85, 128.72, 128.42, 12...
Embodiment 2
[0047] General Synthesis of 2-Alkoxycarbonyl-1,4-Benzoquinones
[0048]
[0049] Silver oxide (3.0mmol) and MgSO 4 (3.0mmol) was added to a solution of 1,4-dihydroxybenzene-2-carboxylate (1.0mmol) in ether (20mL), the resulting reaction solution was stirred at 25°C for 1.5h, filtered, and the filtrate was concentrated to obtain the product.
[0050]
[0051] Yield 95%; 1 HNMR (400MHz, CDCl 3 )δ=7.14(d, J=1.6Hz, 1H), 6.88-6.83(m, 2H), 3.93(s, 3H); 13 CNMR (100Hz, CDCl 3 ) δ 186.86, 182.86, 163.21, 137.07, 137.02, 136.63, 136.23, 53.02.
[0052]
[0053] Yield 96%; 1 HNMR (400MHz, CDCl 3 )δ=7.09(d, J=1.6Hz, 1H), 6.85-6.80(m, 2H), 4.37(q, J=7.1Hz, 2H), 1.36(t, J=7.1Hz, 6H); 13 CNMR (100Hz, CDCl 3 ) δ 187.11, 183.19, 162.24, 137.03, 136.67, 135.91, 135.79, 61.98, 13.99.
[0054]
[0055] Yield 94%; 1 HNMR (400MHz, CDCl 3 )δ=7.09(d,J=1.6Hz,1H),6.83-6.82(m,2H),4.26(t,J=6.4Hz,2H),1.91-1.74(m,2H),1.01(t,J =7.2Hz,3H); 13 CNMR (100Hz, CDCl 3 ) δ 186.88, 182.87,...
Embodiment 3
[0069] Synthesis of 7-phenyl-2-naphthol
[0070]
[0071] Phenylboronic acid (0.24g, 2.0mmol), Ba(OH) 2 ·8H 2 O (0.9g, 2.8mmol), Pd (PPh 3 ) 4 (0.06g, 0.05mmol), 1,4-dioxane (10mL), H 2 O (3mL) and 7-bromo-2-naphthol (1.5mmol) were refluxed under nitrogen for 24h, cooled to room temperature, 1,4-dioxane was removed, and the resulting phase was dissolved in DCM (30mL), 1M hydrochloric acid Wash (20mL×3), wash with saturated brine (20mL×2), Na 2 SO 4 After drying, the solvent was removed, and the residue was purified by silica gel column chromatography (PE / DCM=2 / 1) to obtain the product.
[0072] Yield 72%; 1 HNMR (400MHz, CDCl 3 )δ=7.88-7.83(m, 2H), 7.79(d, J=8.8Hz, 1H), 7.72(d, J=8.2Hz, 2H), 7.61(d, J=8.5Hz, 1H), 7.48( t, J=7.6Hz, 2H), 7.38(t, J=7.2Hz, 1H), 7.21-7.20(m, 1H), 7.12-7.10(m, 1H), 5.06(s, 1H); 13 CNMR (100Hz, CDCl 3 )δ 153.84, 141.30, 139.41, 134.99, 129.76, 128.96, 128.42, 128.25, 127.56, 124.46, 123.61, 117.93, 109.91.
[0073] Synthesis of 6-pheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com