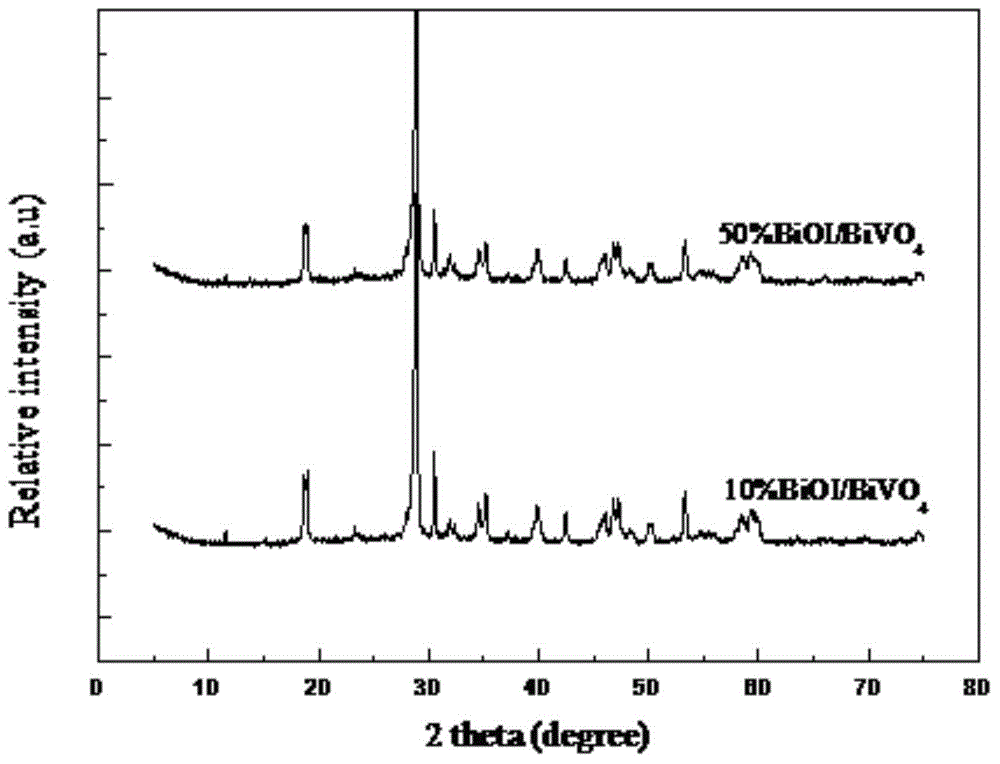
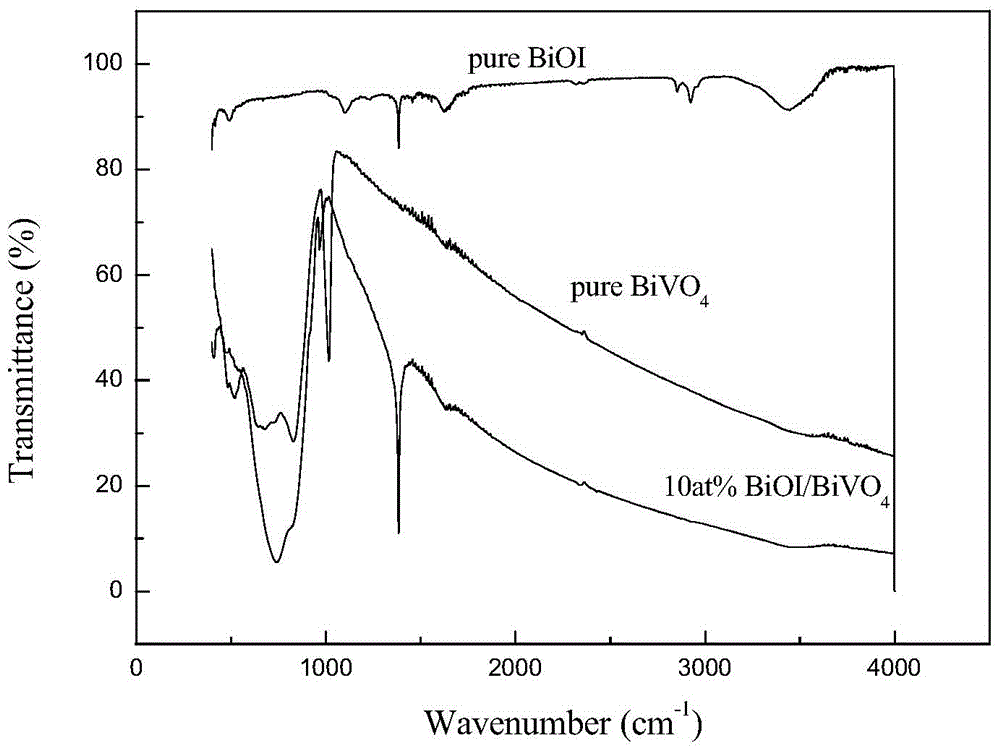
Bismuth oxyiodide-bismuth vanadium oxide heterojunction photocatalyst and preparation method thereof
A technology of bismuth oxyiodide and photocatalyst, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of poor adsorption and low separation efficiency of photogenerated carriers, and achieve low cost, The preparation method is simple and easy to operate, and the effect of inhibiting recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The first step: take 0.01mol (4.8507g) Bi(NO 3 ) 3 ·5H 2 O and 0.02mol (4.2g) C 6 h 8 o 7 ·H 2 Dissolve O in 50mL of 1mol / L dilute nitric acid, stir for 30min to dissolve completely, add ammonia water to adjust the pH value to 7, and record it as solution A;
[0038] The second step: take 0.01mol (1.1698g) NH 4 VO 3 and 0.02mol (4.2g) C 6 h 8 o 7 ·H 2 Dissolve O in 50mL of distilled water, stir for 30min to dissolve completely, and record it as solution B;
[0039] Step 3: After stirring solution B at 80°C for 40 minutes, the solution will turn from orange yellow to dark green, then slowly add solution A and mix to form solution C;
[0040] Step 4: Take 0.001mol (0.4851g) Bi(NO 3 ) 3 ·5H 2 O is dissolved in 15ml ethylene glycol, and is recorded as D solution;
[0041] Step 5: Take 0.001mol (0.166g) of KI and dissolve it in 50mL of distilled water, and record it as E solution;
[0042] Step 6: After mixing solution D and solution E for 15 hours, add it to ...
Embodiment 2
[0049] The first step: take 0.01mol (4.8507g) Bi(NO 3 ) 3 ·5H 2 O and 0.02mol (4.2g) C 6 h 8 o 7 ·H 2 Dissolve O in 50mL of 1mol / L dilute nitric acid, stir for 30min to dissolve completely, add ammonia water to adjust the pH value to 7.1, and record it as solution A;
[0050] The second step: take 0.01mol (1.1698g) NH 4 VO 3 and 0.02mol (4.2g) C 6 h 8 o 7 ·H 2 Dissolve O in 50mL of distilled water, stir for 30min to dissolve completely, and record it as solution B;
[0051] Step 3: After stirring solution B at 80°C for 40 minutes, the solution will turn from orange yellow to dark green, then slowly add solution A and mix to form solution C;
[0052] The fourth step: take 0.005mol (2.4254g) Bi(NO 3 ) 3 ·5H 2 O is dissolved in 20ml ethylene glycol, and is recorded as D solution;
[0053] Step 5: Dissolve 0.005mol (0.83g) KI into 50mL distilled water, and record it as E solution;
[0054] Step 6: After mixing solution D and solution E for 18 hours, add it to the ...
Embodiment 3
[0061] The first step: take 0.01mol (4.8507g) Bi(NO 3 ) 3 ·5H 2 O and 0.01mol (2.1g) C 6 h 8 o 7 ·H 2 Dissolve O in 40mL of 1mol / L dilute nitric acid, stir for 20min to dissolve completely, add ammonia water to adjust the pH value to 6.5, and record it as solution A;
[0062] The second step: take 0.01mol (1.1698g) NH 4 VO 3 and 0.01mol (2.1g) C 6 h 8 o 7 ·H 2 O was dissolved in 40mL of distilled water, stirred for 20min to completely dissolve, and recorded as B solution;
[0063] Step 3: After stirring solution B at 60°C for 50 minutes, the solution will turn from orange yellow to dark green, then slowly add solution A and mix to form solution C;
[0064] The fourth step: take 0.0005mol (0.2425g) Bi(NO 3 ) 3 ·5H 2 O is dissolved in 10ml of ethylene glycol, which is recorded as D solution;
[0065] Step 5: Dissolve 0.0005mol (0.083g) KI into 40mL distilled water, and record it as E solution;
[0066]Step 6: After mixing solution D and solution E for 12 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com