Rapid high-selectivity fluorine ion colorimetric probe and preparation method therefor
A technology of colorimetric probes and fluoride ions, which is applied in chemical instruments and methods, color-changing fluorescent materials, color/spectral characteristic measurement, etc., can solve the problems of limited application and development, poor reproducibility of fluoride-ion selective electrodes, and sensitivity of capillary electrophoresis Low-level problems, to achieve the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] (Scheme 1) Dissolve 174mg (1.0mmol) 2-cyanobenzothiazole, 361mg (1.0mmol) 4-tert-butyldiphenylsilyloxybenzaldehyde and 50μL triethylamine in 20mL ethanol, at 25°C After stirring and reacting for 8 hours, the crude product was obtained by filtration under reduced pressure, and then separated by column chromatography using a mixed system of dichloromethane and petroleum ether (v / v, 1:1) to obtain 310 mg of a light yellow pure product with a yield of 60%.
[0026] (Scheme 2) Dissolve 174mg (1.0mmol) 2-cyanobenzothiazole, 542mg (1.5mmol) 4-tert-butyldiphenylsilyloxybenzaldehyde and 50μL triethylamine in 20mL ethanol, at 25°C After stirring and reacting for 8 hours, the crude product was obtained by filtration under reduced pressure, and then separated by column chromatography using a mixed system of dichloromethane and petroleum ether (v / v, 1:1) to obtain 372 mg of a light yellow pure product with a yield of 72%.
[0027] 1 H-NMR (400MHz, CDCl 3 )δ(*10 -6 ):1...
Embodiment 2
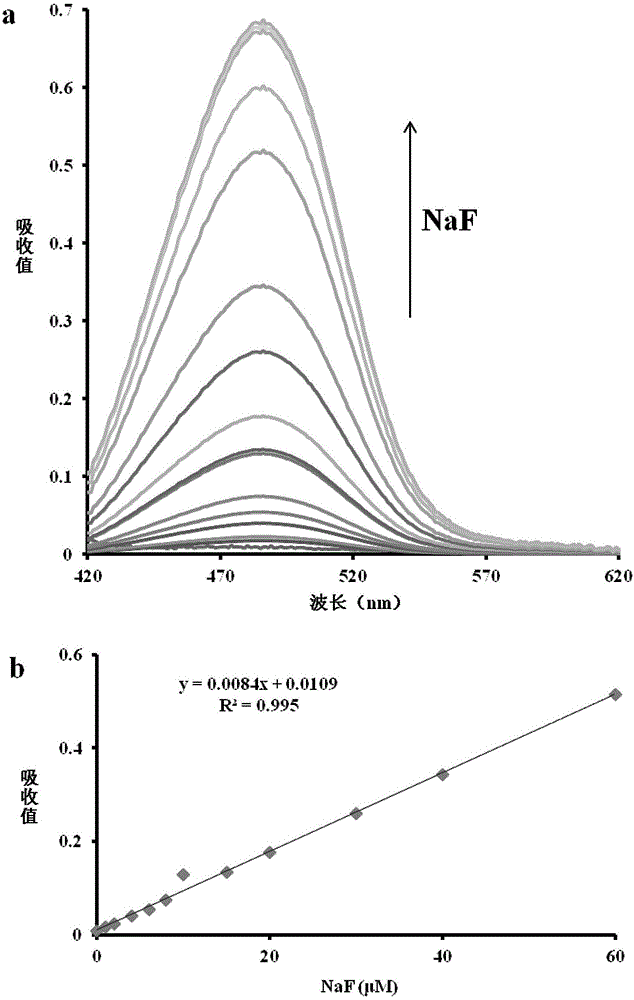
[0029] The inventor of the present invention has carried out following test: (a) the impact of different concentration NaF (0~200 μ M) on probe (10 μ M) absorption spectrum; 2,4,6,8,10,15,20,30,40,60μM). The above measurements were performed in acetonitrile, and all spectral measurements were made at 25°C after NaF addition for 20 min. See results figure 1 .
[0030] From figure 1 It can be seen that with the increase of NaF concentration in the probe solution, the absorption spectrum gradually increases, and has a good linear relationship with the absorption value in the range of 0-60 μM NaF concentration. Therefore, the probe of the present invention can more accurately determine the content of fluoride ions in the blood sample to be tested or in the environment.
Embodiment 3
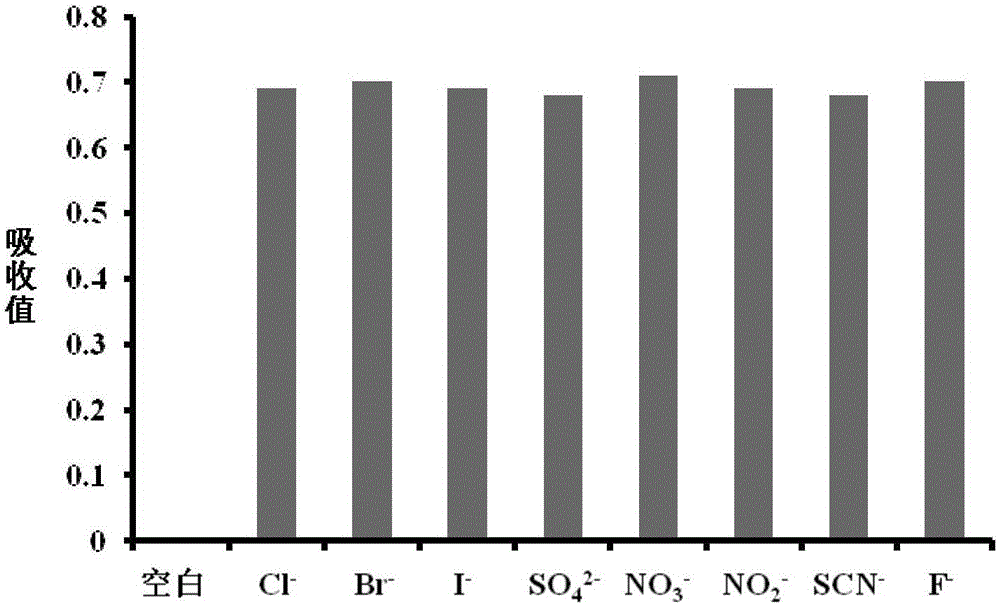
[0032] Effect of different analytes (100 μM) on the absorption spectra of probes (10 μM). Analytes include: Chloride Cl - , bromide ion Br - , iodide ion I - , Sulfate ion SO 4 2- , nitrate ion NO 3 - , nitrite ion NO 2 - , Thiocyanate ion SCN - and fluoride ion F - , and their concentration is 100 μM. All test conditions were performed in acetonitrile and all spectra were measured at 25°C after 20 min of analyte addition. Pipette 50 μL of the probe stock solution (1 mM) into a 5 mL colorimetric tube, then add 3 mL of acetonitrile, then pipette 50 μL of the above analyte stock solution (10 mM) into the colorimetric tube, and then dilute to 5 mL with acetonitrile. Shake well, let it stand for 20min, then measure. The result is as figure 2 shown.
[0033] From figure 2 It can be seen that the probe has high selectivity to fluoride ions and can specifically react with fluoride ions. In acetonitrile solution, compared with other analytes, the absorption spectrum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com