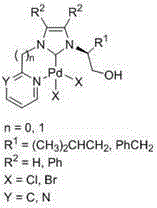
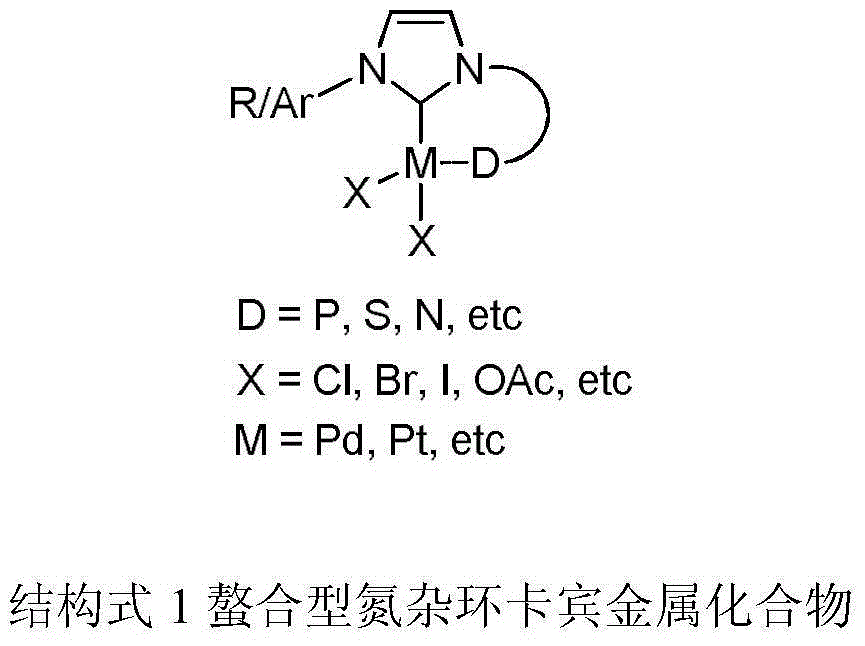
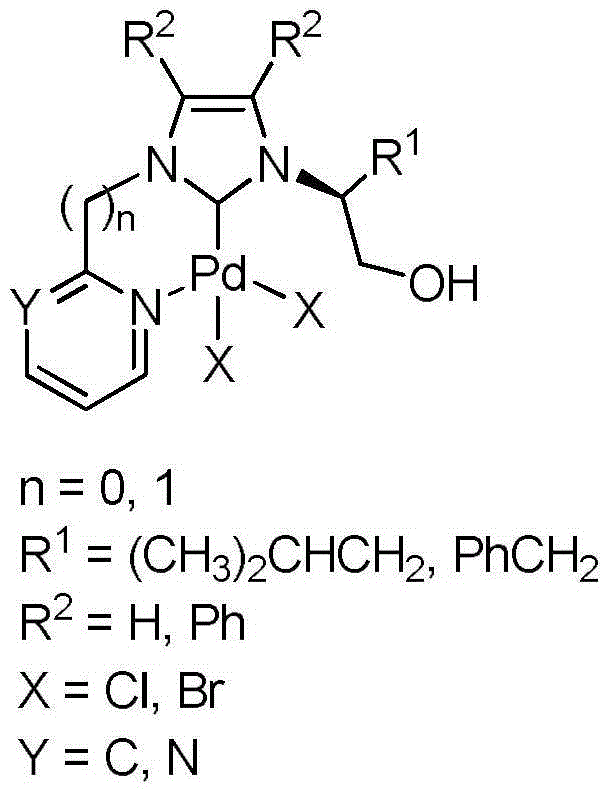
Chelate-type N-heterocyclic carbene palladium compound containing heteroaromatic group and preparation method of same
A nitrogen heterocyclic carbene and palladium compound technology, applied in the field of organic compound synthesis, can solve the problems of polluted products, difficult immobilization, no active groups, etc., and achieve the effects of high-efficiency catalytic performance, high catalytic yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) In a 250mL three-necked flask, L-leucinol (60mmol, 7.03g) and ammonium chloride (60mmol, 3.21g) were dissolved in 120mL methanol, and under cooling in an ice-water bath, the mass percentage was added dropwise with a constant pressure funnel successively 36% formaldehyde solution (60 mmol, 4.6 mL) and 40% mass percent glyoxal solution (60 mmol, 7.6 mL). After the dropwise addition was completed, the temperature was raised to 60° C. to react for 5 h, and the reaction solution was spin-dried to obtain a reddish-brown viscous liquid. The above reddish-brown viscous liquid was dissolved in 150mL NaOH solution (2M), extracted with dichloromethane (20mL×3), the organic phases were combined, and anhydrous Na 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain pure (S)-2-(1-imidazolyl)-4-methylpentanol (7.57 g, yield 75%).
[0028] (2) Add (S)-2-(1-imidazolyl)-4-methylpentanol (5.0mmol, 0.84g), 2-bromopy...
Embodiment 2
[0032] Step (1) is the same as Example 1
[0033] (2) Add (S)-2-(1-imidazolyl)-4-methylpentanol (5.0 mmol, 0.84 g), 2-chloropyrimidine (6 mmol, 0.69 g) and 15 mL of toluene into a 50 mL round bottom flask , heated to 110 °C for 72 h, cooled to room temperature, and the viscous yellow solid formed in the system was collected, separated and purified by column chromatography to obtain N-(1-hydroxy-4-methyl-2-pentyl)-N'- Pure (2-pyrimidinyl)-imidazolium chloride salt (1.12 g, 79% yield). 1 HNMR (400MHz, DMSO): δ10.34(s, 1H, CHinimidazole), 9.08(d, J=4.9Hz, 2H, CHinpyrimidine), 8.55(t, J=1.8Hz, 1H, CHinimidazole), 8.22(t, J=1.7Hz, 1H, CHinimidazole), 7.79 (t, J=4.9Hz, 1H, CHinpyrimidine), 5.47 (t, J=5.4Hz, 1H, CH 2 OH), 4.75-4.71 (m, 1H, NCH), 3.68-3.77 (m, 2H, CH) 2 OH),1.99-1.92(m,1H,CH 2 CH(CH 3 ) 2 ),1.67-1.60(m,1H,CH 2 CH(CH 3 ) 2 ),1.43-1.36(m,1H,CH 2 CH(CH 3 ) 2 ),0.93(d,J=6.5Hz,3H,CH 2 CH(CH 3 ) 2 ),0.88(d,J=6.6Hz,3H,CH 2 CH(CH 3 ) 2 ))ppm. 13 CNMR (100MH...
Embodiment 3
[0037] (1) In a 250mL three-necked flask, dissolve L-phenylalaninol (60mmol, 9.07g) and ammonium chloride (60mmol, 3.21g) with 120mL methanol, under ice-bath cooling, use a constant pressure funnel to drop the mass successively 36% formaldehyde solution (60 mmol, 4.6 mL) and 40 mass percent glyoxal solution (60 mmol, 7.6 mL). After the dropwise addition was completed, the temperature was raised to 60° C. to react for 5 h, and the reaction solution was spin-dried to obtain a reddish-brown viscous liquid. The above yellow oily viscous substance was dissolved in 150 mL NaOH solution (2M), extracted with dichloromethane (20 mL×3), the organic phases were combined, and anhydrous Na 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain pure (S)-2-(1-imidazolyl)-3-phenylalaninol (9.71 g, yield 80%).
[0038] (2) Add (S)-2-(1-imidazolyl)-3-phenylalaninol (5.0 mmol, 1.01 g), 2-bromopyridine (3 mL) and 2 mL of toluene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com