Circulating tumor cell detection kit, preparing method thereof and application thereof
A technology for detecting kits and tumor cells, applied in the field of circulating tumor cell detection kits and their preparation, can solve the problems of low capture efficiency and short shelf life of the detection kits, and achieve long storage time, easy storage, and increased collision probability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
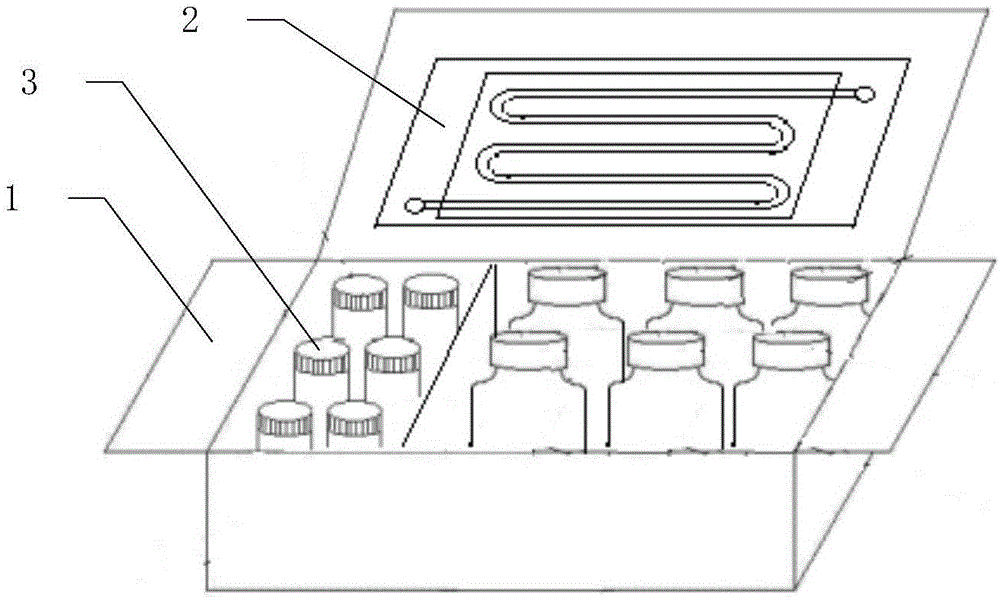
[0047] Such as figure 1 As shown, the circulating tumor cell detection kit includes a box body 1, a microfluidic chip 2 arranged in the box body 1, and a reagent bottle set 3; the reagent bottle set includes a cell recognition probe, red blood cell lysate , Washing Solution, Blocking Solution, Fixative Solution, Permeabilization Solution, and Fixed Cell Stain.
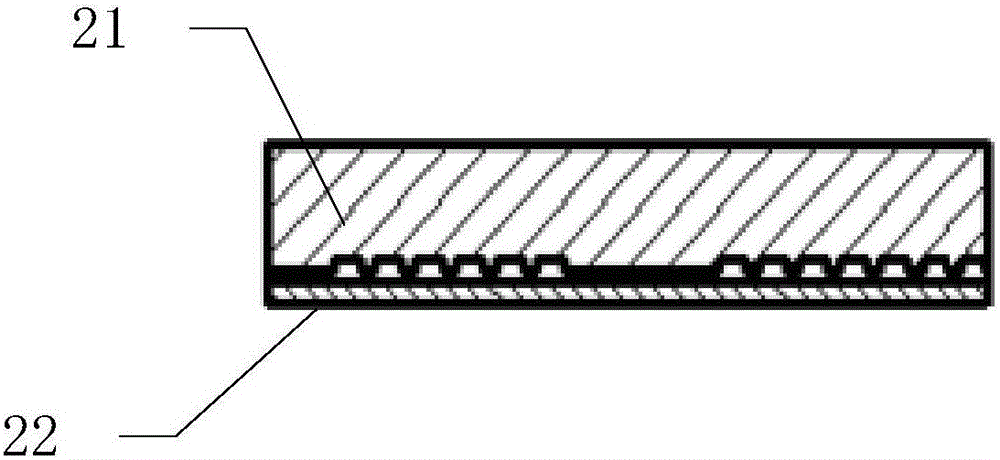
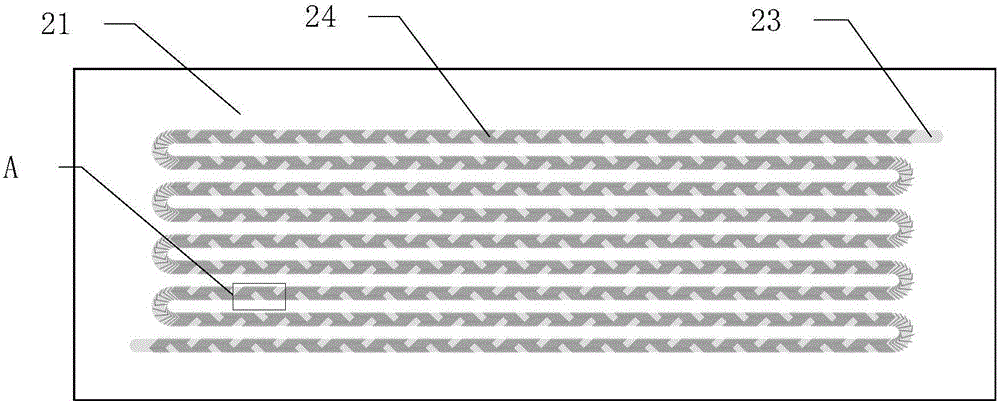
[0048] Such as figure 2 As shown, the microfluidic chip 2 is composed of a PDMS substrate 21 and a slide glass 22, as image 3 As shown, microchannels 23 are distributed on the surface of the PDMS substrate 21, the microchannels 23 are single channels, and are arranged in 9 rows in a continuous S-shape, and the bottom surface of the microchannels 23 has a periodic interlaced mixing structure 24, Such as Figure 4 As shown, the interlaced mixing structure 24 is a herringbone structure.
[0049] Such as Figure 5 As shown, after the PDMS substrate 21 is bonded to the slide glass 22, the microfluid channel 25 is for...
Embodiment 2
[0056] The difference from Example 1 is that the reagent bottle set includes a cell recognition probe reagent bottle 31 , a red blood cell lysate reagent bottle 32 , a cleaning solution reagent bottle 33 , a blocking solution reagent bottle 34 and an unfixed cell staining reagent bottle.
[0057] In addition, the slide 22 is a polylysine-modified slide from ThermoScientific, and the capture probe 26 also modifies the surface of the slide 22 in addition to the surface of the microchannel 23 .
[0058] The non-fixable cell stain includes Cy3-labeled first single-stranded polynucleotide and APC-labeled anti-CD45. Sequentially select the filter slides of CY5 and TRITC to observe under a fluorescent microscope, the first single-stranded polynucleotide labeled with Cy3 is red, and the APC-labeled anti-CD45 is green.
Embodiment 3
[0059] The preparation method of the microfluidic chip described in embodiment 3
[0060] The preparation method of the microfluidic chip 2 described in Example 1 and Example 2 is as follows: After the PDMS substrate 21 is treated with oxygen in a plasma cleaner for 15 seconds, it is soaked in a 4% 3-aminopropyltriethoxysilane solution. Soak for 1 hour to generate amino groups on the surface, and then wash with ultrapure water to remove excess reagents.
[0061] After drying with nitrogen gas, the amino-modified PDMS substrate 21 was attached to a clean glass slide 22, and placed in an oven at 80° C. for 2 hours, and after returning to room temperature, one-step loading and capturing anti-probe 26 was carried out. The specific method is: mix 500 μL of amino-modified first single-stranded polynucleotide with DMSO at a volume ratio of 3:2, and then mix the mixed solution with BS3 (disuccinimide suberate sodium salt) aqueous solution at a volume ratio of 1:1. After mixing, pass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bump height | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com