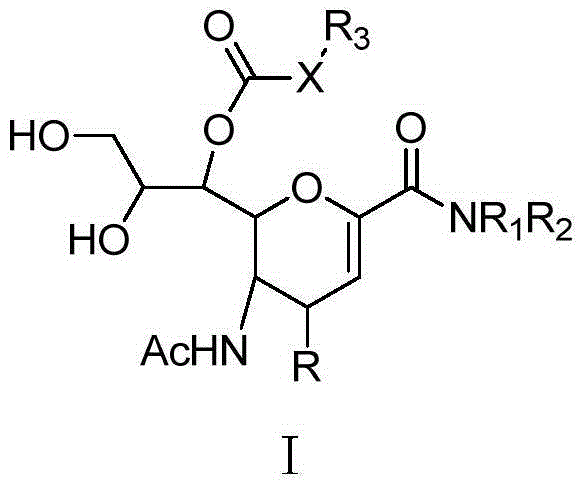
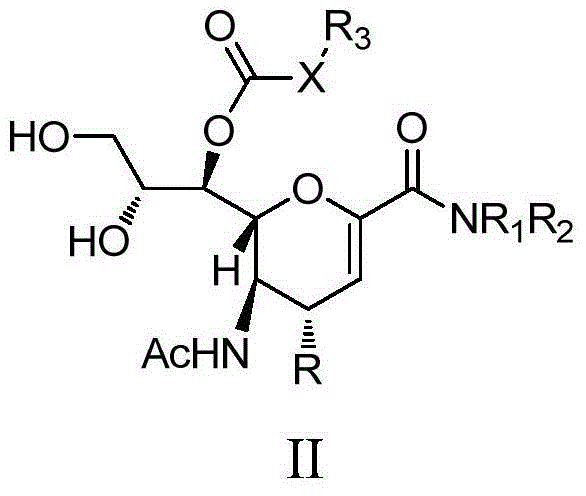
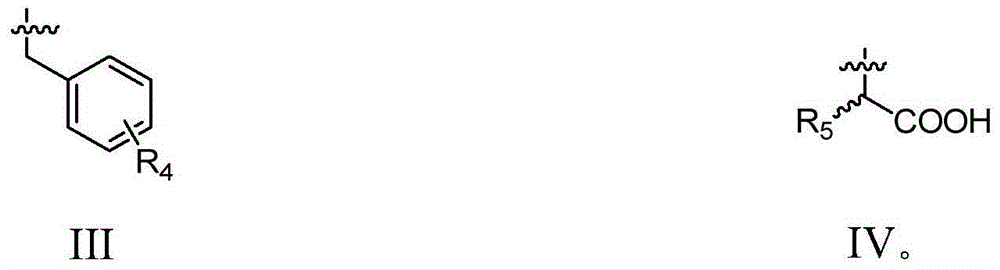Sialic acid analogues or pharmaceutically acceptable salts and application thereof
A technology of analogs and sialic acid, applied in the field of sialic acid analogs or their pharmaceutically acceptable salts and their applications, can solve the problems of poor oral bioavailability, rapid excretion, etc., achieve superior application prospects, and inhibit influenza virus , good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of compound 17a comprises the following steps:
[0067] (1) Preparation of compound 2.
[0068] Add 5 g of cesium carbonate to 10 ml of aqueous solution of sialic acid 1 (10 g, 32.4 mmol), adjust the pH value to 7-8, spin the solvent under reduced pressure, add 30 mL of DMF and 6 mL of benzyl bromide, react at room temperature for 24 hours, suction filter, and the filtrate Pour it into 1000mL of dichloromethane, a lot of solids precipitate out, and filter it with suction to obtain compound 2.
[0069] The characterization data of this compound 2 is: 1HNMR (400MHz, D 2 O,ppm)δ7.31-7.41(m,5H),5.22(dd,J=22.4,12.8Hz,2H),4.04(m,1H),3.99(dd,J=10.4,1.6Hz,1H), 3.80(d,J=2.8Hz,1H),3.77(m,1H),3.70(m,1H),3.62(dd,J=10.8,5.2Hz,1H),3.48(dd,J=9.2,1.2Hz ,1H),2.22(dd,J=12.8,5.2Hz,1H),1.91(dd,J=12.8,11.2Hz,1H); LC-MSm / z398[M+H] + .
[0070] (2) Preparation of Compound 3.
[0071] Dissolve 0.31 mol of compound 2 in pyridine (250 mL, 3.1 mol), add a catalytic amount of...
Embodiment 2
[0102] The preparation of compound 17b, compound 17b was prepared by the method for preparing compound 17a in Example 1, wherein, after obtaining compound 8 in Example 1, the following steps were performed:
[0103] (8) Preparation of Compound 9b.
[0104] Compound 8 (0.2mmol), EDCI (0.3mmol), Et 3 N (0.3 mmol) was dissolved in 5 mL of dichloromethane, an equivalent amount of glycine was added, and stirred at room temperature for 24 h. After TLC showed that compound 8 disappeared, dichloromethane was removed by rotary evaporation under reduced pressure, and compound 9b was obtained by column chromatography.
[0105] (9) Preparation of compound 10b: same as compound 10a, except that compound 9a is replaced by compound 9b.
[0106] (10) Preparation of compound 11b: Same as compound 11a, except that compound 10a is replaced by compound 10b.
[0107] (11) Preparation of compound 13b: Same as compound 13a, except that N-(3-aminopropyl)-3,4-dihydroxybenzamide is replaced by N-(3-a...
Embodiment 3
[0112] For the preparation of compound 17c, compound 17c was prepared by the method for preparing compound 17a in Example 1, wherein, after obtaining compound 8, the following steps were performed:
[0113] (8) Preparation of Compound 9c.
[0114] Dissolve compound 8 (0.2mmol), EDCI (0.3mmol), Et3N (0.3mmol) in 5mL of dichloromethane, add an equivalent amount of aminoisopropionic acid, stir at room temperature for 24h, after TLC shows that the starting material 8 disappears, spin down under reduced pressure Dichloromethane was distilled off and further purified by column chromatography to obtain compound 9c.
[0115] (9) Preparation of compound 10c: same as compound 10a, except that compound 9a is replaced by compound 9c.
[0116] (10) Preparation of compound 11c: Same as compound 11a, except that compound 10a is replaced by compound 10c.
[0117] (11) Preparation of compound 13c: Same as compound 13a, except that N-(3-aminopropyl)-3,4-dihydroxybenzamide is replaced by N-(3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com