Hydrazone bond-containing block copolymer having targeting antitumor activity and preparation thereof, and applications of block copolymer as antitumor drug carrier
An anti-tumor activity, block copolymer technology, applied in the field of polymer chemistry, can solve the problems of strong cardiotoxicity and limitation of doxorubicin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
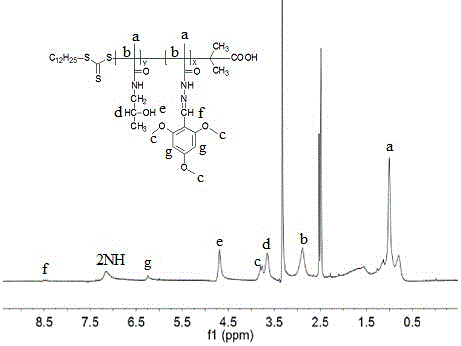
[0071] Embodiment 1, the preparation of two-block polymer copolymer A
[0072] Preparation of 4-nitrophenyl methacrylate: 4-nitrophenol (0.5g, 3.6mmol) and triethylamine (0.437g, 4.32mmol) were dissolved in 15ml of THF, cooled to 0°C in an ice-water bath, Methacryloyl chloride (0.417ml, 4.32mmol) was dissolved in 10ml of THF and slowly added dropwise to the above solution. Reaction 12~15h. After the reaction, the precipitate produced in the filter solution was washed once with a small amount of distilled water, anhydrous MgSO 4 Dry and serve. Yield 95%.
[0073] 1 HNMR (400MHz, CDCl 3 ,δ,ppm):8.29(d, J =7.2Hz,2H,–OC 6 h 2 H 2 NO 2 ),7.43(d, J =7.2Hz,2H,–OC 6 H 2 h 2 NO 2 ),6.39(s,1H,CH H =C(CH 3 )CO–),5.84(s,1H,C H H=C(CH 3 )CO–),2.07(s,3H,CHH=C(C H 3 )CO–).
[0074] compound Preparation: Dissolve trithioester RAFT reagent (7 mg, 25 μmol) and 4-nitrophenyl methacrylate (0.312 g, 1.5 mmol) in N,N-dimethylformamide (DMF), add Nitroisobutyronitrile (AIB...
Embodiment 2
[0080] Embodiment 2, the preparation of triblock macromolecule copolymer B
[0081] compound , The preparation: with embodiment 1.
[0082] compound Preparation: Weigh folic acid (441.4mg, 1.0mmol), cyclohexylcarbodiimide (247.6mg, 1.2mmol) and N-hydroxysuccinimide (138.1mg, 1.2mmol), dissolve in 30mL di In methyl sulfoxide, react in the dark at 45~55°C for 6~8 hours. After all the folic acid is converted into folic acid activated ester, add allylamine (571mg10mmol) to the reaction solution, and react in the dark at -5~5°C for 12~ 15h. Add acetone to precipitate, filter with suction, and dry in a vacuum oven to obtain (321.2 mg, yield 65%).
[0083] 1 HNMR(400MHz,DMSO-d6,δ,ppm):8.62(s,1H,–CH–ofheterocyclic),7.61(d, J =8.6Hz,2H,–CH 2 NHC 6 H 2 h 2 CONHCH(COOH)CH 2 CH 2 CO–), 6.64 (d, J =8.6Hz,2H,–CH 2 NHC 6 h 2 H 2 CONHCH(COOH)CH 2 CH 2 CO–),5.88(m,1H,–NHCH 2 C H =CH 2 ),5.28(dd, J =27.0,13.9Hz,2H,–NHCH 2 CH=C H 2 ),4.46(s,2H,–C H 2 NHC 6 h 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com