A kind of chitosan nanoparticle and preparation method for improving oral colonic absorption of insulin
A technology of chitosan nanoparticles and insulin, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of insulin oral bioavailability, unstable nanoparticles, Inability to protect substances and other issues, to achieve good physical and chemical stability, protective activity, and increased interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
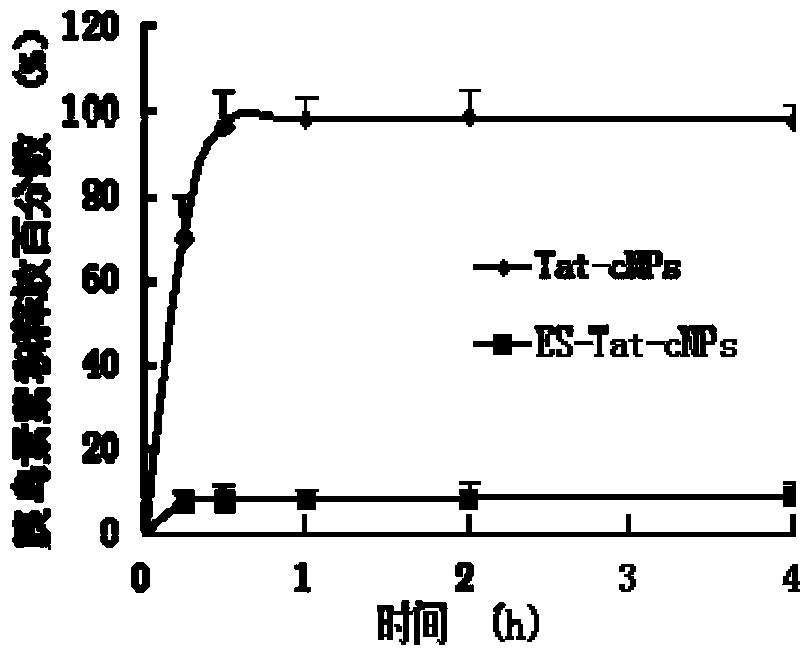
Image
Examples
Embodiment 1
[0080] Take by weighing 8 mg of chitosan powder whose molecular weight is 100,000 Daltons and have a deacetylation degree of 95%, dissolve it in 0.2% acetic acid solution of 4 ml, adjust the pH value to 5.5 with 1mol / L sodium hydroxide solution, and call it Solution A. 2 mg of Tat (amino acid sequence GRKKRRQRRRP) was weighed, dissolved in solution A and called solution B, and solution B was stirred on a magnetic stirrer at room temperature. Weigh 4.2 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2.3 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanopart...
Embodiment 2
[0082]Take by weighing 8 mg of chitosan powder with a molecular weight of 50,000 Daltons and a degree of deacetylation of 85%, dissolve it in 0.2% acetic acid solution of 4 ml, adjust the pH value to 6.0 with 1mol / L sodium hydroxide solution, called Solution A. 2.3 mg of Tat (amino acid sequence GRKKRRQRRRP) was weighed, dissolved in solution A and called solution B, and solution B was stirred on a magnetic stirrer at room temperature. Weigh 4 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanoparticle suspe...
Embodiment 3
[0084] Take by weighing 8 mg of chitosan powder whose molecular weight is 100,000 Daltons and whose degree of deacetylation is 85%, dissolve it in 4 ml of 0.2% acetic acid solution, adjust the pH value to 5.5 with 1mol / L sodium hydroxide solution, called Solution A. 2 mg of Tat (amino acid sequence GRKKRRQRRRP) was weighed, dissolved in solution A and called solution B, and solution B was stirred on a magnetic stirrer at room temperature. Weigh 4 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2.3 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanoparticle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com