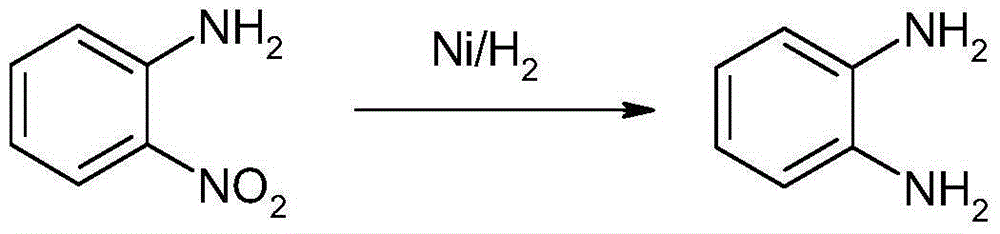
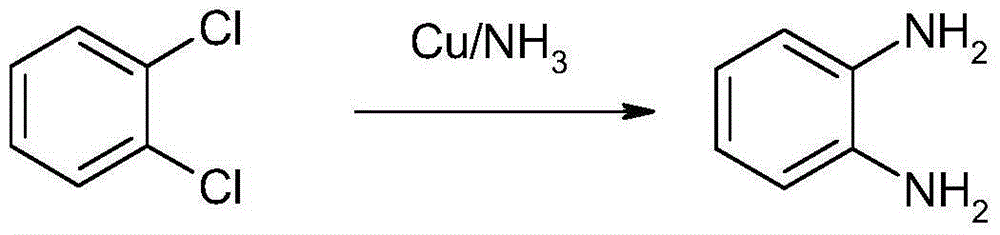
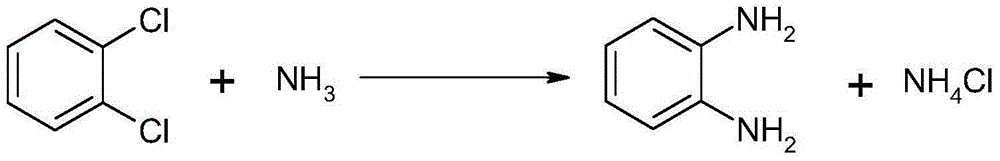
Method for synthesizing and preparing o-phenylenediamine from orthodichlorobenzene
A technology for o-dichlorobenzene and o-phenylenediamine, which is applied in the field of synthesizing o-phenylenediamine, can solve the problems of increased equipment investment, potential safety hazards, long reaction time, etc., so as to improve reaction efficiency and reaction yield, and reduce reaction time. Difficulty, the effect of promoting the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 1L autoclave, add 100g of o-dichlorobenzene, 2g of cuprous iodide, 0.5g of triphenylphosphine and 200g of liquid ammonia, control the reaction temperature at 85-100°C, and the reaction pressure at 5-7MPa. After 6 hours of reaction, Then, the oil phase was separated at 100° C., and 66.9 g of white o-phenylenediamine was obtained by vacuum distillation, with a yield of 90.5% and a purity of 99.3% (GC). The combination of cuprous iodide and triphenylphosphine forms copper diamine (I) and copper triamine (I) complexes with higher activity, lower reaction enthalpy, mild reaction conditions, and increased yield. The product is less, and the purity of the product is high.
Embodiment 2
[0024] In a 1L autoclave, add 100g of o-dichlorobenzene, 5g of cuprous iodide, 4g of triphenylphosphine and 200g of liquid ammonia, control the reaction temperature at 85-100°C, and the reaction pressure at 5-6MPa. After reacting for 10 hours, The oil phase was separated at 90° C., and 68.0 g of white o-phenylenediamine was obtained by vacuum distillation, with a yield of 92% and a purity of 99.0% (GC).
Embodiment 3
[0026] In a 1L autoclave, add 100g of o-dichlorobenzene, 3g of cuprous iodide, 1g of triphenylphosphine and 200g of liquid ammonia, control the reaction temperature at 85-100°C, and the reaction pressure at 5-6MPa. After reacting for 10 hours, then The oil phase was separated at 90° C., and 68.0 g of white o-phenylenediamine was obtained by vacuum distillation, with a yield of 92% and a purity of 99.1% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com