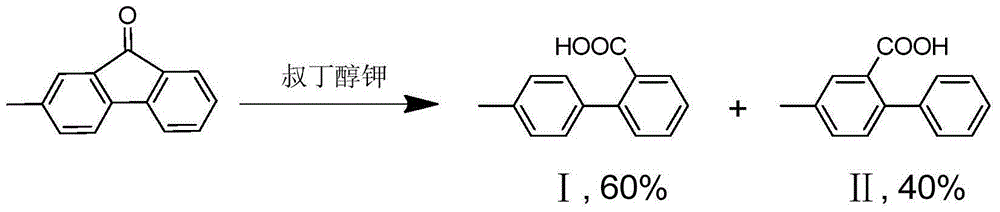
Asymmetric diphenic acid isomeride separation method
A technology of isomers and diphenylformic acid, which is applied in the field of separation of asymmetric biphenylformic acid isomers, can solve the problems of cumbersome operation, long encapsulation time, general separation effect, etc., and achieve mild reaction conditions, Easy to operate and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put the mixed acid (4.24g, 20mmol) and potassium hydroxide (1.12g, 20mmol) in a reaction flask, add 100mL of water, stir it magnetically to dissolve it, then heat it in an oil bath, and distill part of the water to make the solution slightly cloudy , and then cooled to 0°C, a white solid was precipitated, filtered, the filter cake was redissolved in water, and hydrochloric acid was added to adjust the pH to 4-6, the solid was precipitated, filtered, and dried to obtain 1.42g of product, the yield was 33.5%, Ⅰ The purity is 76%.
Embodiment 2
[0026] Put mixed acid (4.24g, 20mmol) and calcium hydroxide (0.74g, 10mmol) in a reaction flask, add 100mL of water, stir magnetically and add an oil bath to make it dissolve, continue to heat up to distill part of the water to make the solution slightly It was turbid, then cooled to 0°C, a white solid was precipitated, filtered, the filter cake was redissolved in water, and hydrochloric acid was added to adjust the pH to 4-6, the solid was precipitated, filtered, and dried to obtain 1.61g of product with a yield of 38.0%. I has a purity of 87%.
Embodiment 3
[0028] Put the mixed acid (6.36g, 30mmol) and aluminum hydroxide (0.78g, 10mmol) in a reaction flask, add 200mL of water, stir magnetically and add an oil bath to make it dissolve, continue to heat up and distill part of the water to make the solution slightly It was turbid, then cooled to 0°C, a white solid was precipitated, filtered, the filter cake was redissolved in water, and the pH was adjusted to 4-6 by adding hydrochloric acid, the solid was precipitated, filtered, and dried to obtain 3.31 g of the product, with a yield of 52.0%. I has a purity of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com