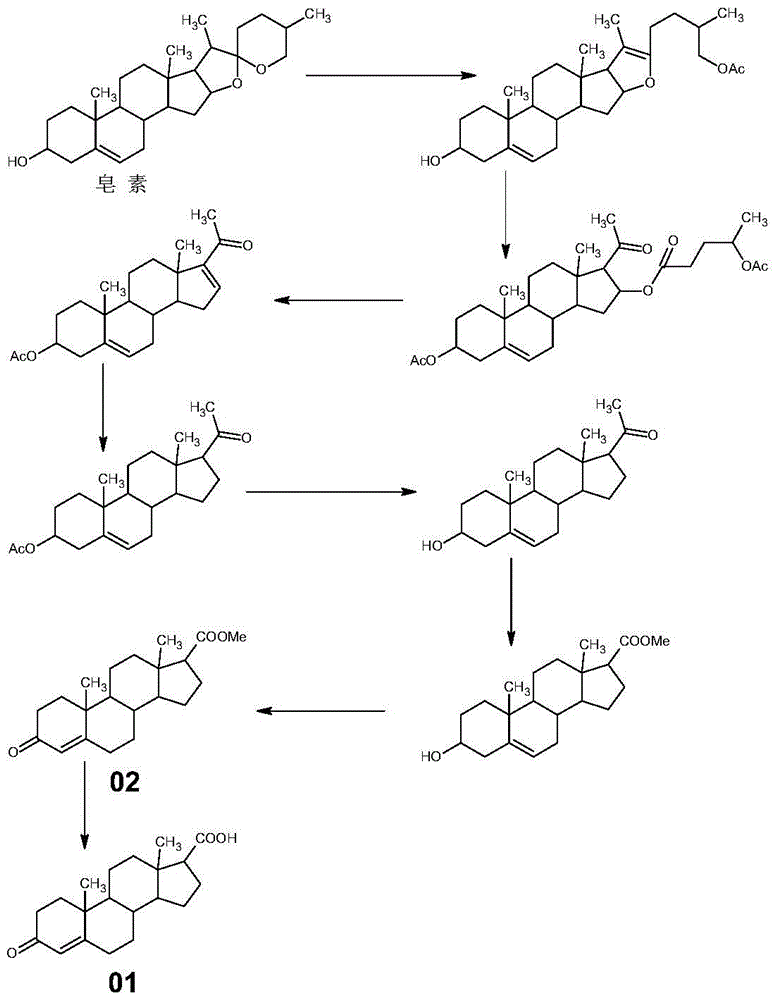
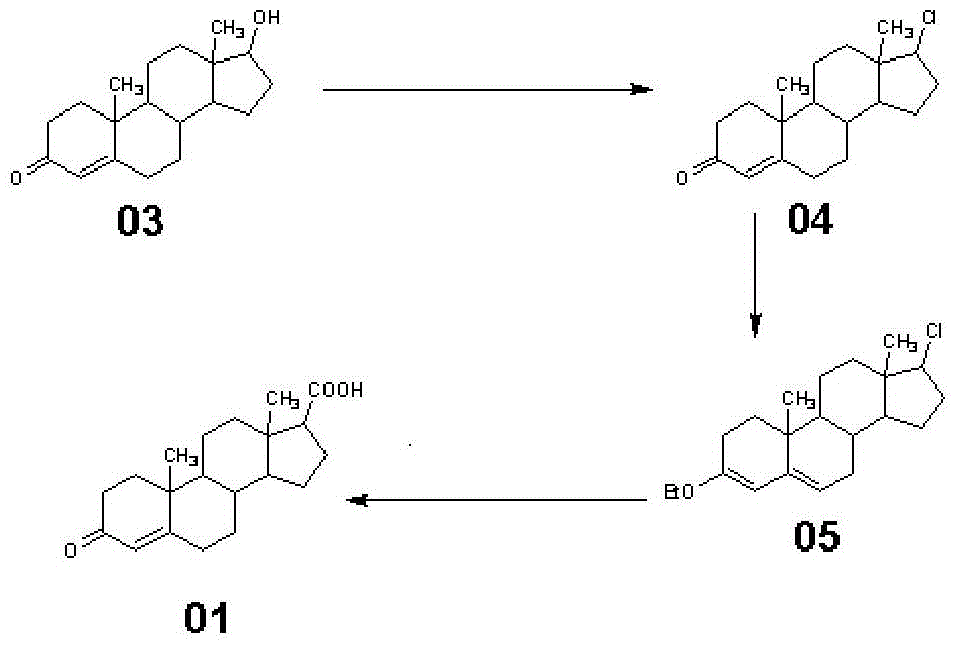
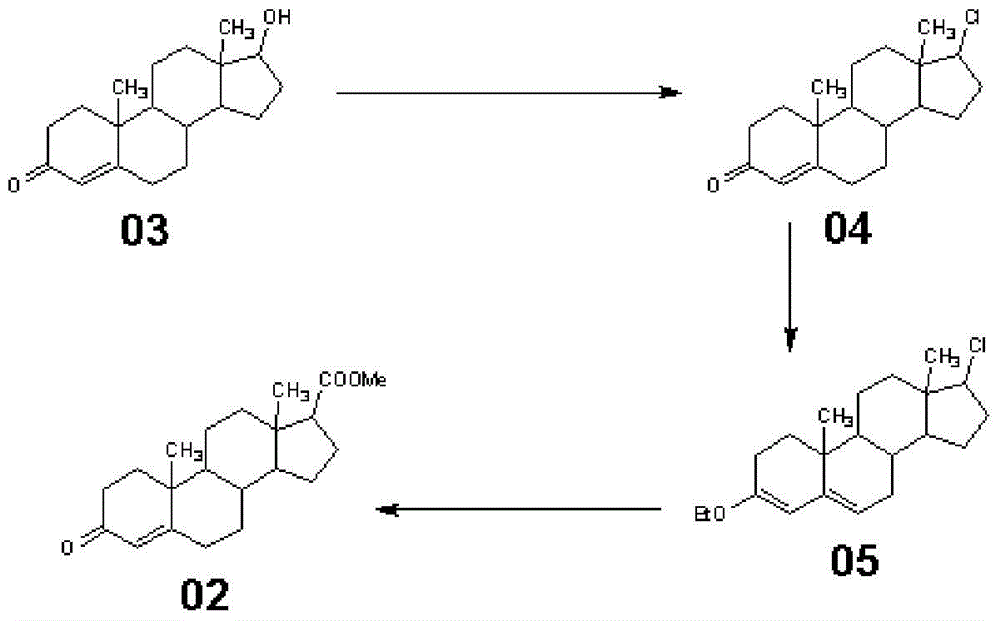
Synthetic method of 3-keto-4-androstene-17β carboxylic acid and its methyl ester
A synthesis method and technology of methyl carboxylate, applied in the field of drug synthesis, can solve the problems of reduced molecular weight, low labor cost, inadequate environmental protection supervision, etc., and achieve the effects of reducing reaction steps, changing synthesis routes, and facilitating industrialized implementation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment is a synthetic method of 3-keto-4-androstene-17β carboxylic acid, which specifically includes the following steps:
[0040] (1) Synthesis of Compound 04
[0041] Add 28g of testosterone and 500mL of anhydrous ether into a 1000mL reaction bottle, then cool down to 0°C, slowly add 12g of thionyl chloride dropwise, and continue the reaction for 5 hours after the dropwise addition. Then it was raised to room temperature overnight. After the reaction was completed, the solvent was spin-dried to obtain 28.28 g of yellow compound 04 with a molar yield of 94.90%.
[0042] (2) Synthesis of Compound 05
[0043] At room temperature, add 500ml tetrahydrofuran and 100ml triethyl orthoformate to a 1000ml reaction glass reaction bottle, stir, add PTS.H 2 O 0.5 g, Compound 04 100 g. Control the temperature at 40°C for 4 hours, spot the plate until the basic reaction is complete; cool down to room temperature, add 1ml of triethylamine, stir for 30 minutes, slowly pour...
Embodiment 2
[0049] This embodiment is a synthetic method of methyl 3-keto-4-androstene-17β carboxylate, and the difference from Example 1 is only that step (3) (c) is different, and (c) of this embodiment is specifically:
[0050] Add compound 05 to the Grignard reagent slowly dropwise into dimethyl carbonate solution (toluene 200ml, add dimethyl carbonate 33g), keep the reaction at 20°C for 4 hours, and spot the plate until the reaction is complete. Slowly add the reaction solution into glacial acetic acid solution (tap water 1000ml + glacial acetic acid 25ml), stop cooling down, concentrate at normal pressure, after concentrating toluene, drop to below 40°C (preferably 30-40°C), filter, wash with water until neutral, Drained and dried to obtain 89.66 g of methyl 3-keto-4-androstene-17β carboxylate, with a molar yield of 90.88%.
Embodiment 3
[0052] The difference between this embodiment and Example 1 is that the amount of thionyl chloride in step (1) is different. In this embodiment, the amount of thionyl chloride is 13g, and finally obtains the yellow compound 0428.09g, and the molar yield is 94.27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com