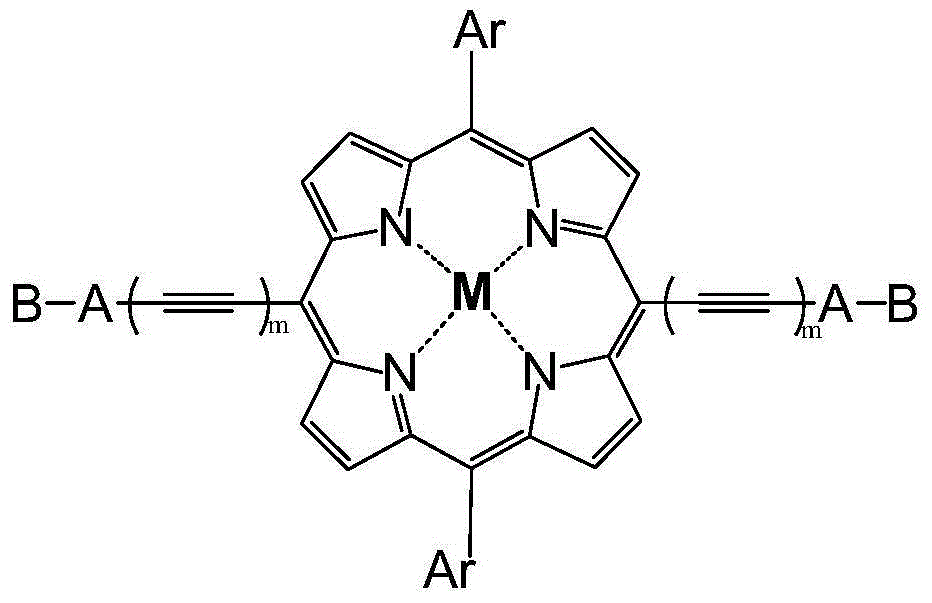
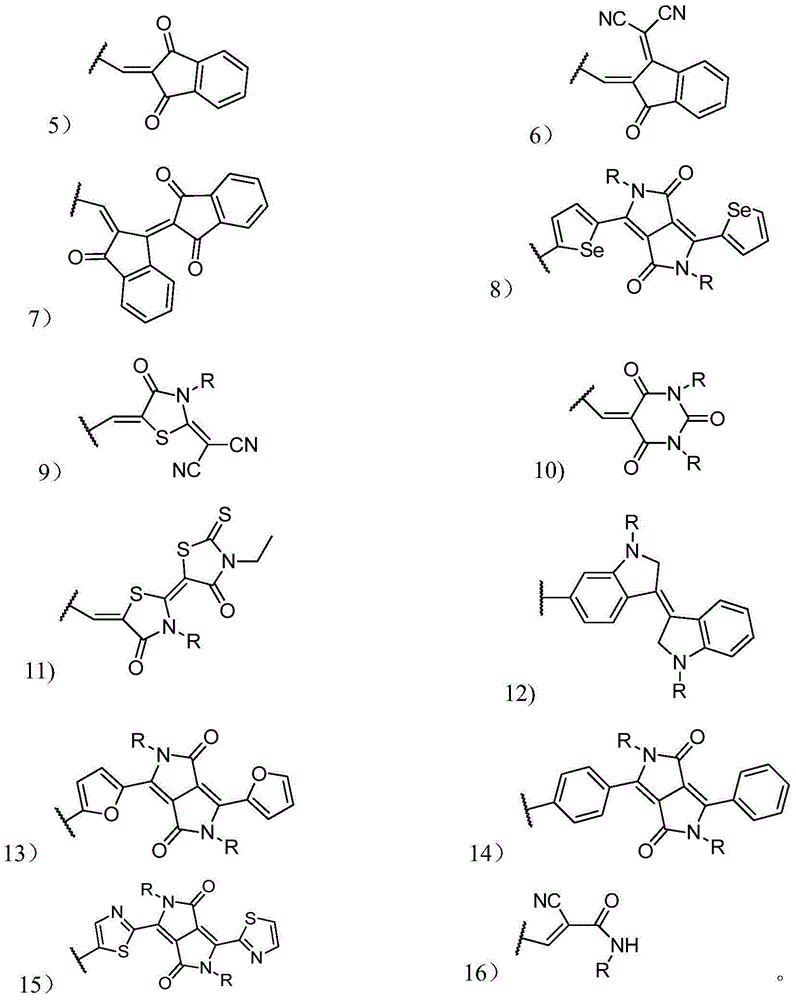
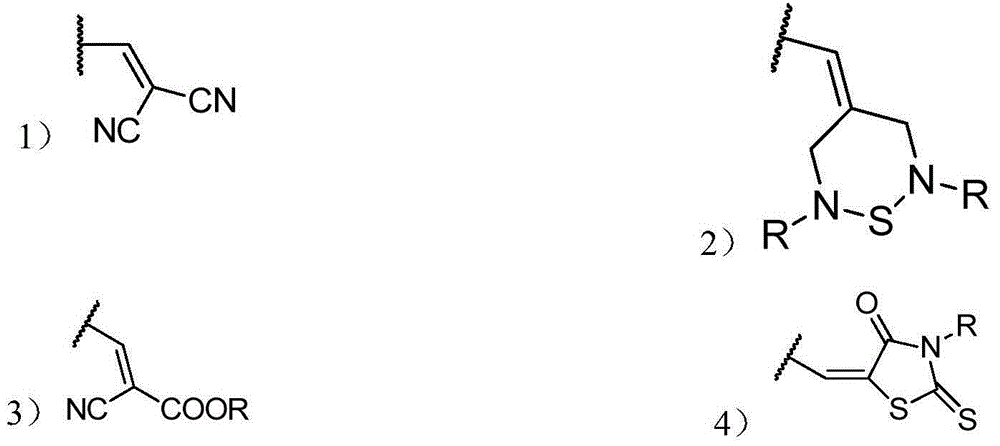Organic small molecular photoelectric functional material, and preparation method thereof
A technology of optoelectronic functional materials and small molecules, which can be used in luminescent materials, photovoltaic power generation, organic chemistry, etc. It can solve the problems of low photoelectric conversion efficiency of organic solar cells, and achieve the effects of strong absorption, improved solubility, and large open circuit voltage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1C16
[0036] The synthesis of embodiment 1C16TPTRD
[0037] Step 1: Synthesis of 15,15-bis(5-(2-hexyldecyl)thiophene)porphyrin
[0038]
[0039] In a 1000mL two-necked round bottom flask, add 5-(2-hexyldecyl)thiophene-2-carbaldehyde (1.632g, 4.86mmol), bipyrromethane (700mg, 4.86mmol) and 500mL of dichloromethane, and ventilate with nitrogen 30 minutes, then add 0.25mL of trifluoroacetic acid, stir the reaction at room temperature for 12 hours, then add 1.8g of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), continue The reaction was stirred for 12 hours, and then 5 mL of triethylamine was added to quench the reaction. After the reaction, the crude product was obtained by column chromatography on silica gel / (dichloromethane as eluent) and spin-dried, and then recrystallized by chloroform / methanol to obtain a dark red solid. 1 H NMR (300MHz, CDCl 3 )δ10.27(s,2H),9.35(q,8H),7.74(d,2H),7.20(d,2H),3.09(d,4H),1.93(m,2H),1.61-1.25(m ,48H), 0.95-0.82(m,12H),-2.96(s,2H).
[0040] S...
Embodiment 2
[0058] Synthesis of Example 2 C16TPPRD
[0059]
[0060] Under the protection of argon, 5,15-bis(acetylene)-10,20-bis(3,5-bis(dodecyloxy)benzene)zinc porphyrin (262mg ,0.2mmol), 4-bromobenzene-2-(3-ethyl)rhodanine (196.2mg, 0.6mmol), anhydrous toluene (10mL), triethylamine (5mL), cuprous iodide (8mg, 0.04 mmol) and tetrakis(triphenylphosphine)palladium (24mg, 0,02mmol), protected from light, stirred and reacted at 80°C for three days. After the reaction was completed, it was cooled to room temperature, washed with water, extracted with toluene, dried over anhydrous sodium sulfate, spin-dried, and passed through a column to obtain a purple-black solid. Mass(MALDI-TOF): Obs.1806.3; Calcd.for C 108 h 134 N 6 o 6 S 4 Zn, 1805.9.
Embodiment 3
[0061] The synthesis of embodiment 3PorEFDPP
[0062]
[0063] Under the protection of argon, 5,15-bis(acetylene)-10,20-bis(3,5-bis(dodecyloxy)benzene)zinc porphyrin (262mg , 0.2mmol), monobromobisfuropyrrolopyrrole diketone (342mg, 0.6mmol), anhydrous toluene (10mL), triethylamine (5mL), cuprous iodide (8mg, 0.04mmol) and tetrakis (tri Phenylphosphine)palladium (24mg, 0,02mmol), protected from light, stirred and reacted at 80°C for three days. After the reaction was completed, it was cooled to room temperature, washed with water, extracted with toluene, dried over anhydrous sodium sulfate, spin-dried, and passed through a column to obtain a purple-black solid. Mass(MALDI-TOF): Obs.2013.6; Calcd.for C 124 h 156 N 8 o 8 S 2 Zn, 2013.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com