Hydrazine near infrared ratio detecting fluorescent probe and application
A ratiometric fluorescent probe and fluorescent probe technology, applied in the field of organic small molecule fluorescent probes, can solve the problems of short wavelength, inability to detect gaseous hydrazine, inability to realize cell imaging, etc., and achieve broad application prospects and good fluorescence ratio response effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
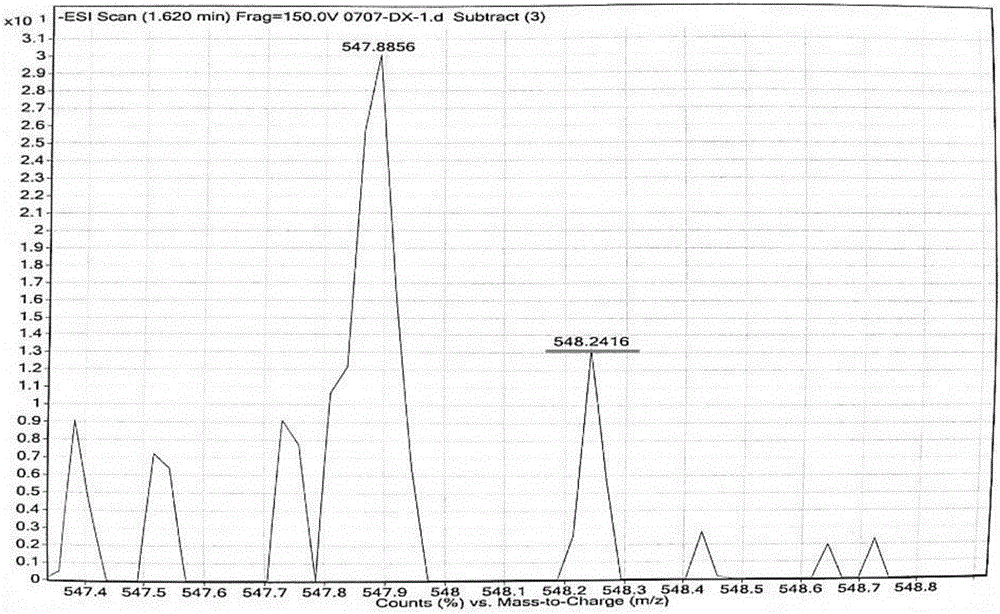
[0028] 2-(2-hydroxyl-4-diethylaminobenzoyl) benzoic acid and 3-acetyl-7 diethylaminobenzoyl coumarin synthesized by a known method are reacted at temperature in concentrated sulfuric acid, and the reaction solution is cooled , add perchloric acid oxidation to obtain 4-(2'-methylcarboxyphenyl)-2-(7'-diethylaminocoumarin)-7-diethylaminobenzopyran perchlorate with red fluorescence salt. specifically is:
[0029] Add 2-(2-hydroxy-4-diethylaminobenzoyl)benzoic acid (3, 5.0 mmol), 3-acetyl-7 diethylamino coumarin (5.0 mmol) into 5 mL of concentrated sulfuric acid , heated to 90°C and controlled for 8 hours, cooled to room temperature, added 10g of ice, then added 1mL of 70% perchloric acid and stirred, a black solid was precipitated, filtered with suction, washed with water three times, and the crude product was recrystallized with ethanol to obtain a dark green solid which was 4 -(2'-methylcarboxyphenyl)-2-(7'-diethylaminocoumarin)-7-diethylaminobenzopyran perchlorate (BPCC), mel...
Embodiment 2
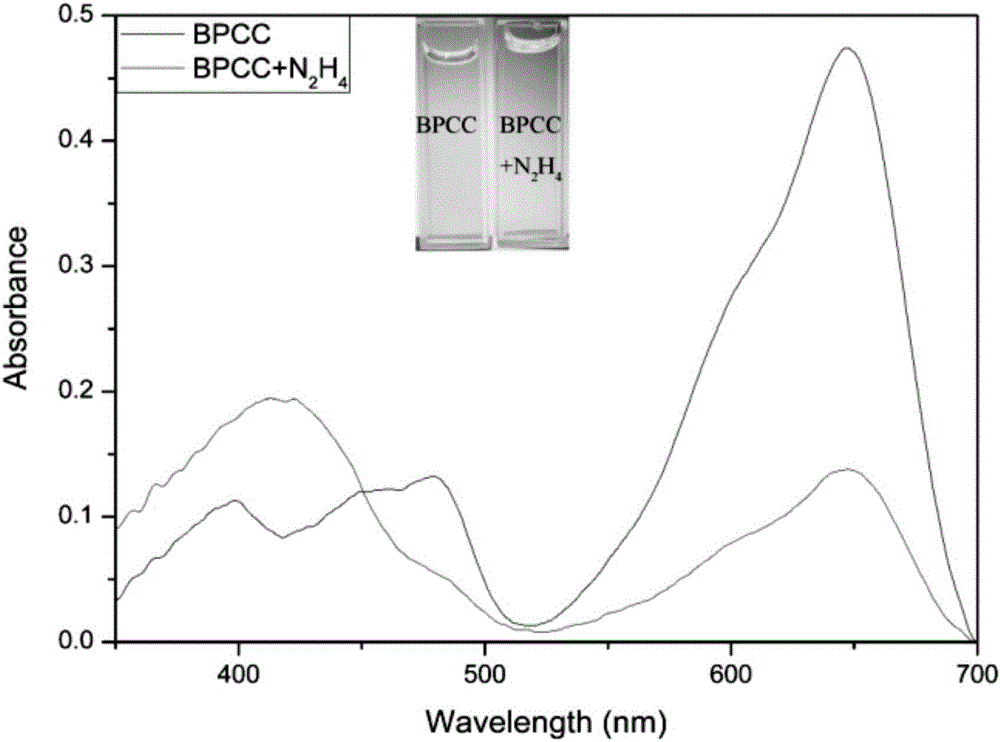
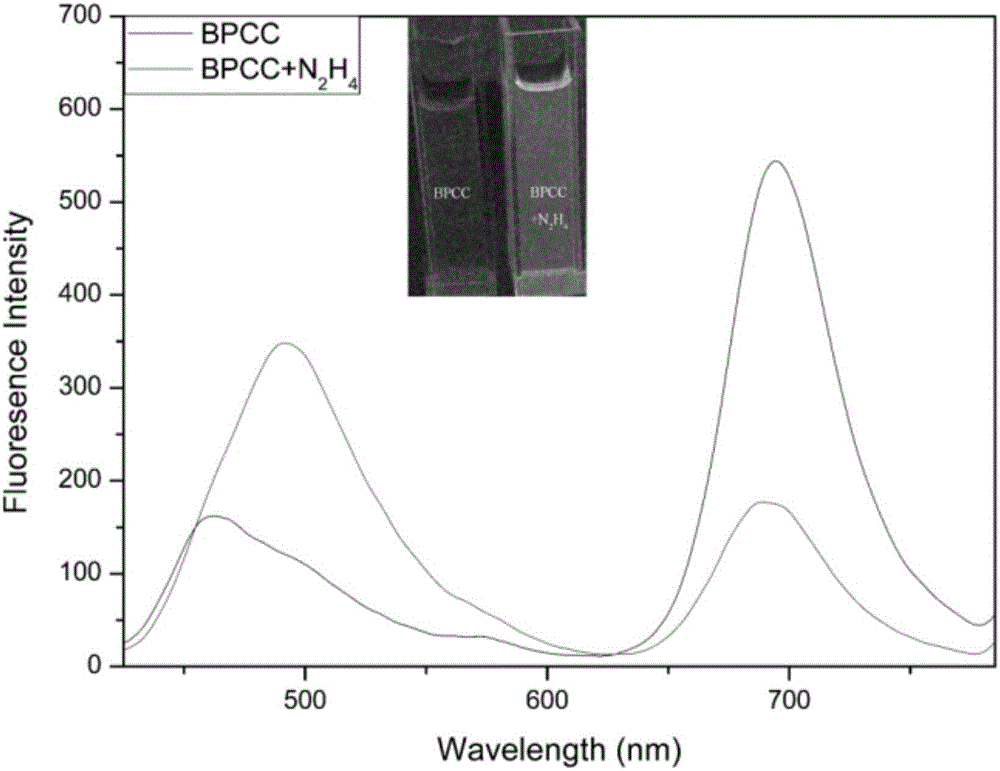
[0037] 10 equivalents of N 2 h 4 ,CH 3 CO 2 - ,Br - ,H 2 PO 4 - , HCO 3 2- ,HPO 4 2- , HSO 3 - ,I - ,NO 2 - ,NO 3 - , SO 3 2- , SO 4 2- , Ca 2+ ,Cu 2+ ,Fe 3+ , K + ,Mg 2+ , Na 2 S, Zn 2+ After 2 hours of action, the UV-visible spectrophotometry and fluorescence spectrophotometry tests were performed, showing that the probes were sensitive to N 2 h 4 There is good selectivity, adding N 2 h 4 The before and after comparison shows that the ultraviolet absorption wavelength and the fluorescence emission wavelength have a blue shift of 167 and 201nm, respectively, which has a very good ratio response. See figure 2 , 3, 4.
Embodiment 3
[0039] Fluorescence test in fetal bovine serum.
[0040] At 37°C, 100% fetal bovine serum was incubated with 5 μM probes and different concentrations of N 2 h 4 (0, 10, 50, 100, 500, 1000μM) were incubated together for 2h. Pure serum has basically no fluorescence in the two channels, the probe shows weak light in the blue channel, and strong fluorescence in the red channel. 2 h 4 As the concentration increases, the blue fluorescence gradually strengthens, and the red fluorescence gradually weakens, see Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com