Application of IMB5046 compound in the preparation of antineoplastic drugs
A technology of anti-tumor drugs and compounds, applied in the field of medicine, can solve problems such as neurotoxicity, poor water solubility, and difficult synthesis and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
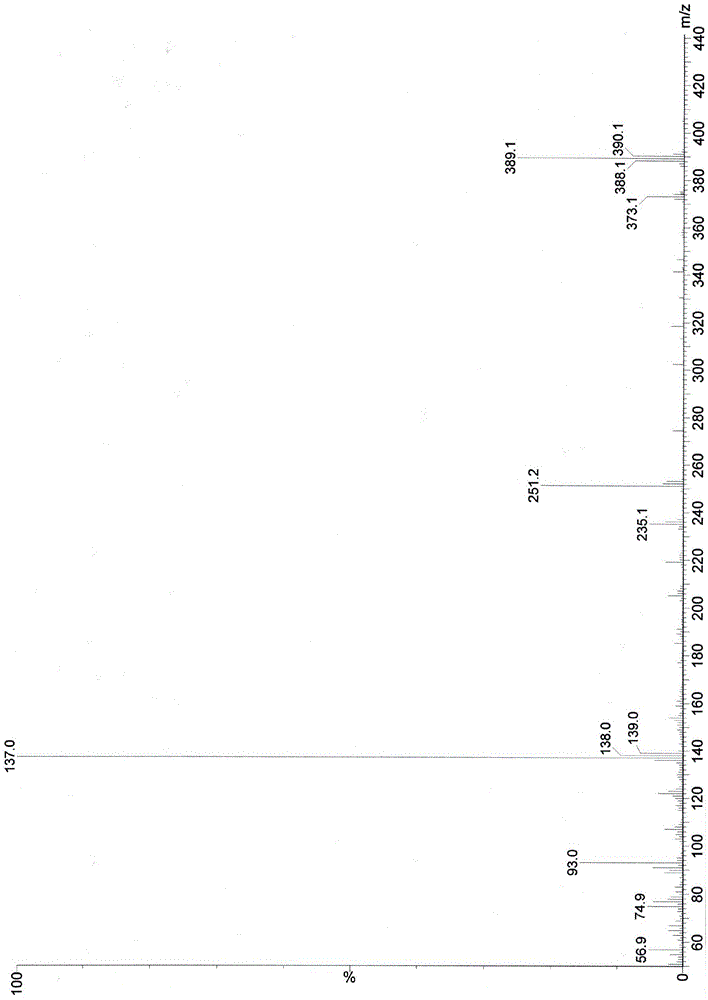
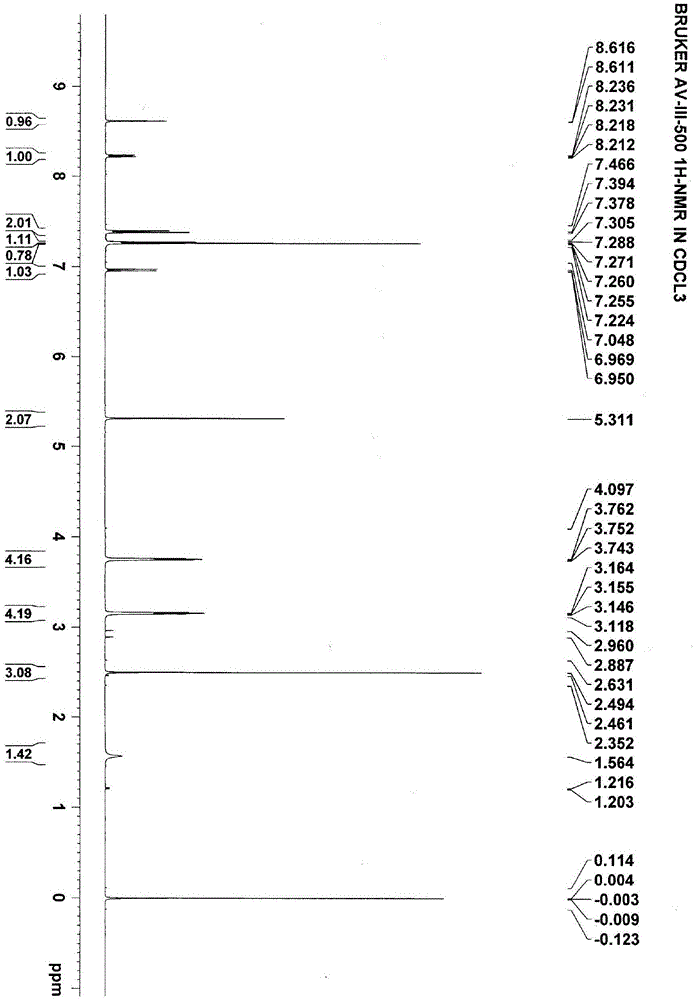
Image
Examples
Embodiment 1
[0100] Embodiment 1: the preparation of chemical formula I compound
[0101] The implementation steps of this embodiment are as follows:
[0102] 2-(4-morpholinyl)-5-nitrobenzoic acid is dissolved in a mixed solvent composed of toluene and dimethylformamide according to a volume ratio of 1:0.1 to obtain a concentration of 2.0% by weight of 2- (4-morpholino)-5-nitrobenzoic acid solution, which was then stirred at a temperature of 60° C. for 0.8 hours, and then added dropwise with a 50% thionyl chloride toluene solution by weight, wherein 2-( The molar ratio of 4-morpholino)-5-nitrobenzoic acid to thionyl chloride is 1:2, and the reaction is continued for 5 hours at the same temperature. Evaporate to dryness under reduced pressure to obtain a kind of evaporated product W1;
[0103] According to the ratio of evaporated dryness W1 in grams to ether in milliliters is 1:50, let the evaporated dryness W1 be recrystallized in ether at room temperature, and the separated crystals are...
Embodiment 2
[0108] Embodiment 2: the preparation of chemical formula I compound
[0109] The implementation steps of this embodiment are as follows:
[0110] 2-(4-morpholinyl)-5-nitrobenzoic acid was dissolved in a mixed solvent composed of toluene and dimethylformamide at a volume ratio of 1:0.2 to obtain a concentration of 1.0% by weight of 2- (4-Morpholinyl)-5-nitrobenzoic acid solution, which was then stirred at a temperature of 70° C. for 1.2 hours, and then added dropwise with a thionyl chloride toluene solution with a concentration of 58% by weight, wherein 2-( The molar ratio of 4-morpholino)-5-nitrobenzoic acid to thionyl chloride is 1:5, and the reaction is continued for 4 hours at the same temperature, and the obtained reaction solution is carried out under the conditions of temperature 42°C and pressure 0.01MPa Evaporate to dryness under reduced pressure to obtain a kind of evaporated product W1;
[0111] According to the ratio of evaporated dryness W1 in grams to ether in m...
Embodiment 3
[0116] Embodiment 3: the preparation of chemical formula I compound
[0117] The implementation steps of this embodiment are as follows:
[0118] 2-(4-morpholinyl)-5-nitrobenzoic acid was dissolved in a mixed solvent composed of toluene and dimethylformamide at a volume ratio of 1:0.1 to obtain a concentration of 1.6% by weight of 2- (4-morpholino)-5-nitrobenzoic acid solution, which was then stirred at a temperature of 50° C. for 1.0 hour, and then added dropwise with a concentration of 70% by weight in thionyl chloride toluene solution, wherein 2-( The molar ratio of 4-morpholino)-5-nitrobenzoic acid to thionyl chloride is 1:1, and the reaction is continued for 8 hours at the same temperature, and the obtained reaction solution is carried out under the conditions of temperature 44°C and pressure 0.004MPa Evaporate to dryness under reduced pressure to obtain a kind of evaporated product W1;
[0119] According to the ratio of evaporated dryness W1 in grams to ether in millil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com