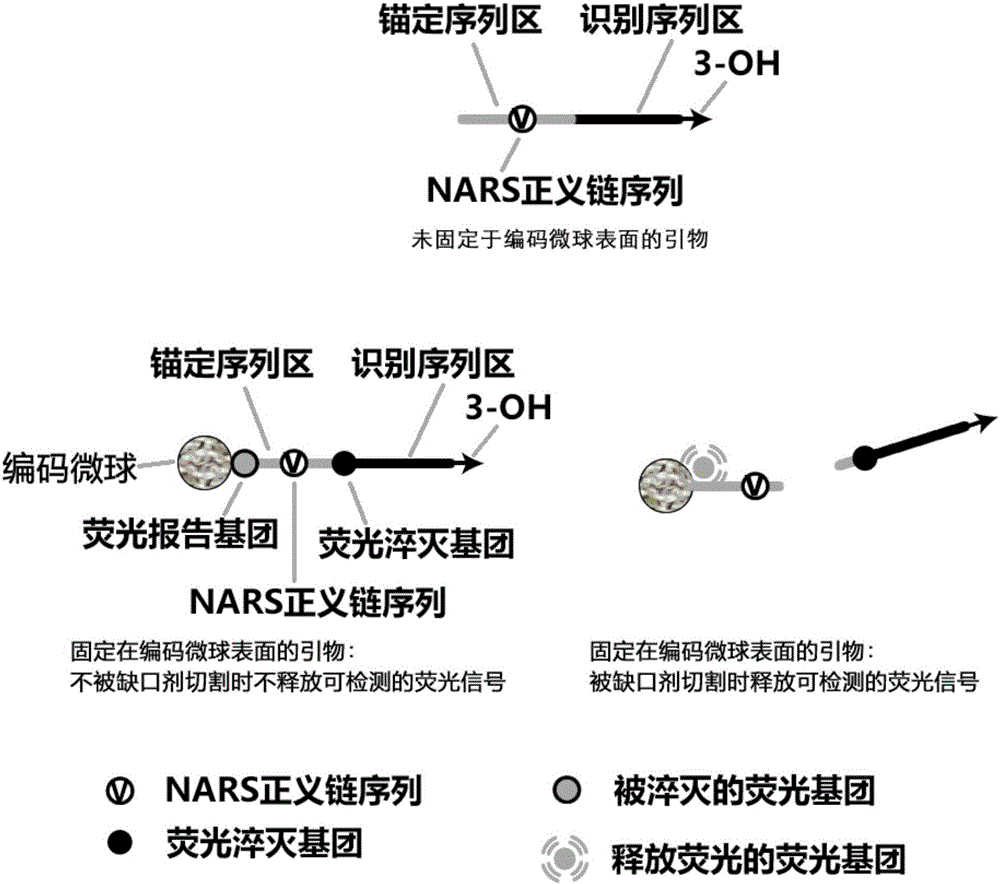
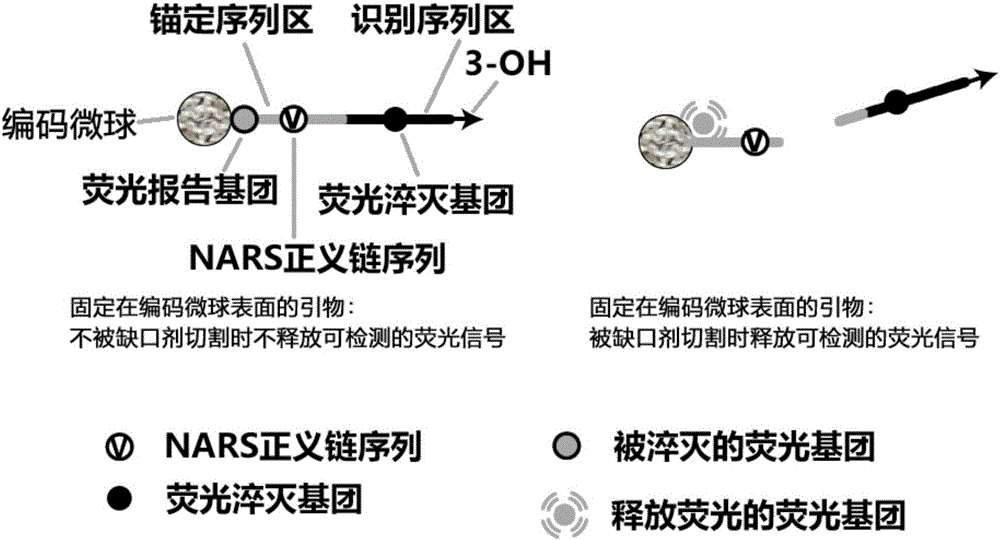
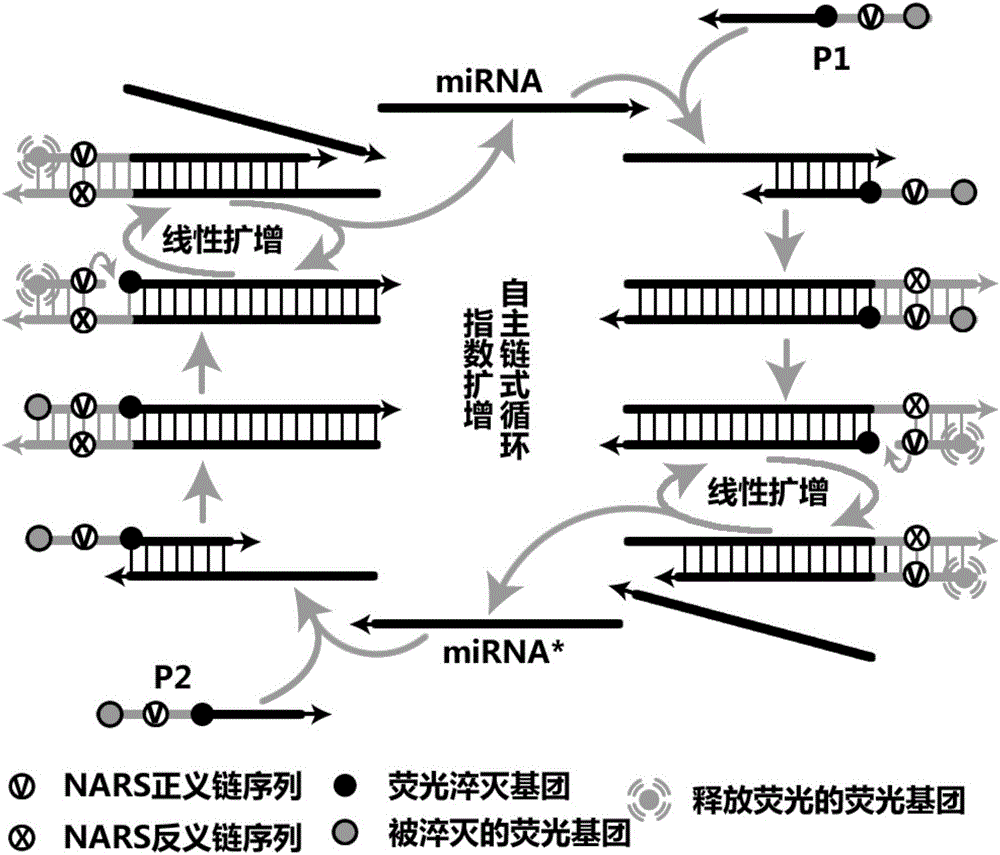
Liquid-stage chip constant-temperature detection method for tiny RNA
A liquid chip and detection method technology, which is applied in the fields of life science and biology, can solve the problems of cumbersome detection steps, long time-consuming, and restrictions on the application of liquid chips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Each type of coding microsphere is immobilized with an upstream primer and / or a downstream primer corresponding to one miRNA target molecule, and six miRNA target molecules are simultaneously detected in a single detection system.
[0095] In this embodiment, the carboxyl-modified MicroPlex commercialized by U.S. Luminex Company is adopted. TM Fluorescence encoded microspheres, the encoded microspheres have 100 kinds of fluorescent encoded microspheres. In theory, when the upstream primer and / or downstream primer corresponding to a miRNA target molecule is immobilized on the surface of each coding microsphere, the upstream primer and / or downstream primer corresponding to each target molecule can label the same fluorescent reporter molecule, Simultaneous detection of 100 miRNA target molecules in a single reaction system. The present invention only uses the detection of six miRNA target molecules as an example to illustrate the detection effect of the present...
Embodiment 2
[0161] Embodiment 2: Each encoding microsphere surface immobilizes the upstream primers and / or downstream primers corresponding to two miRNA target molecules, and simultaneously detects six miRNA target molecules in a single detection system.
[0162] In this embodiment, the carboxyl-modified MicroPlex commercialized by U.S. Luminex Company is adopted. TM Fluorescence encoded microspheres, the encoded microspheres have 100 kinds of fluorescent encoded microspheres. In theory, when the upstream primers and / or downstream primers corresponding to two miRNA target molecules are immobilized on the surface of each encoding microsphere, the primers corresponding to the above two target molecules label two different fluorescent reporter molecules, but different encoding microspheres The fixed primers can be labeled with the same fluorescent reporter molecules, and 200 miRNA target molecules can be detected simultaneously in a single reaction system. The present invention only uses th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com