Enzyme-promoting chemiluminiscence substrate using alkaline phosphatase
An enzymatic chemiluminescence and phosphatase technology, applied in the field of immunological detection, can solve the problems of slow light emission and low luminescence intensity, and achieve the effects of long duration, high sensitivity and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the preparation of enzymatic chemiluminescent substrate (APSH)
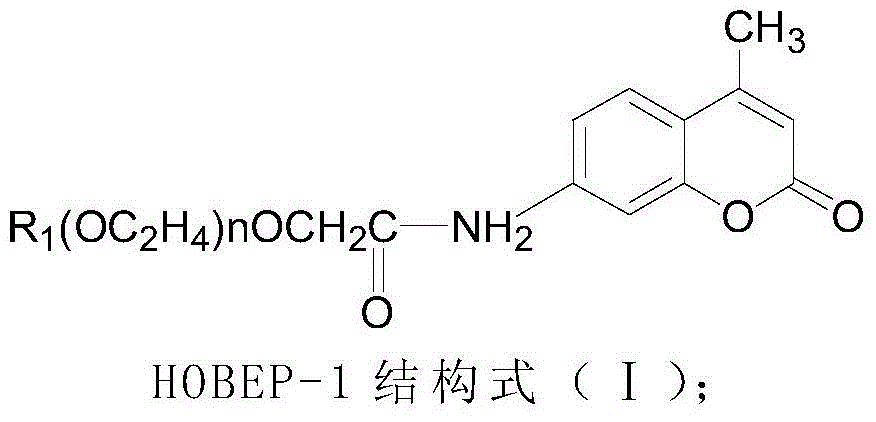
[0038] (1) Materials: 2-amino-2-methyl-1-propanol, AMPPD, MgS0 4 and ZnCl 2 All are commercially available and chemically pure; ProClin300 is a product of Supelco Company in the United States; the luminescence enhancer HOBEP-1 is obtained by coupling fatty alcohol polyoxyethylene ether carboxylate sodium with coumarin fluorescent compounds, and the luminescence enhancer HOBEP-2 is It is obtained by coupling sodium fatty alcohol polyoxyethylene ether carboxylate with fluorescein-based fluorescent compounds, and the luminescence enhancer HOBEP-3 is obtained by coupling sodium fatty alcohol polyoxyethylene ether carboxylate with naphthalimide fluorescent compounds. The enhancer HOBEP-4 is obtained by coupling sodium fatty alcohol polyoxyethylene ether carboxylate with rhodamine fluorescent compounds, and the luminescence enhancer HOBEP-5 is obtained by coupling sodium fatty alcohol polyoxyethyl...
Embodiment 2
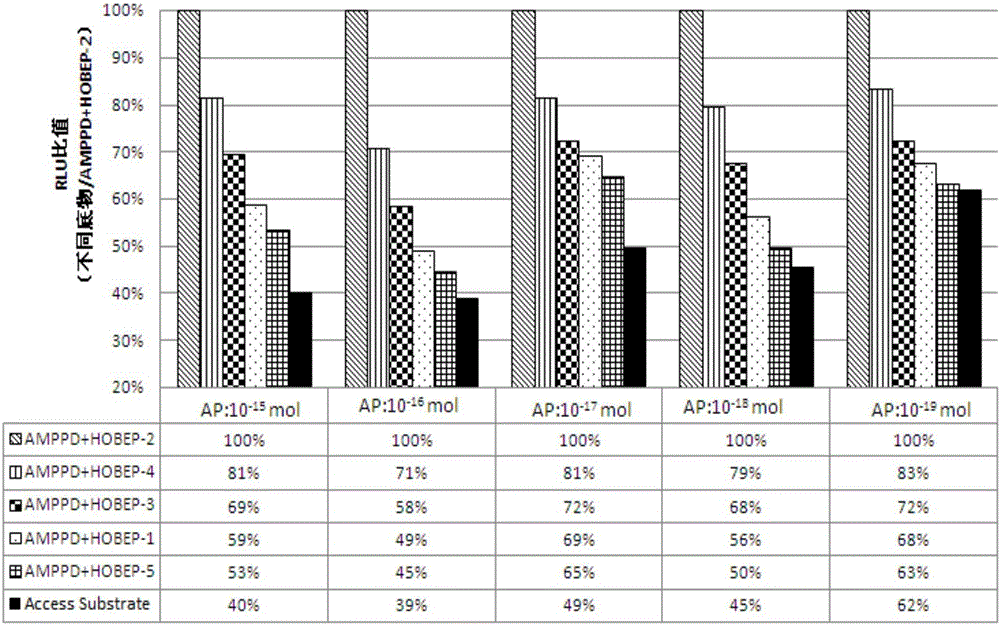
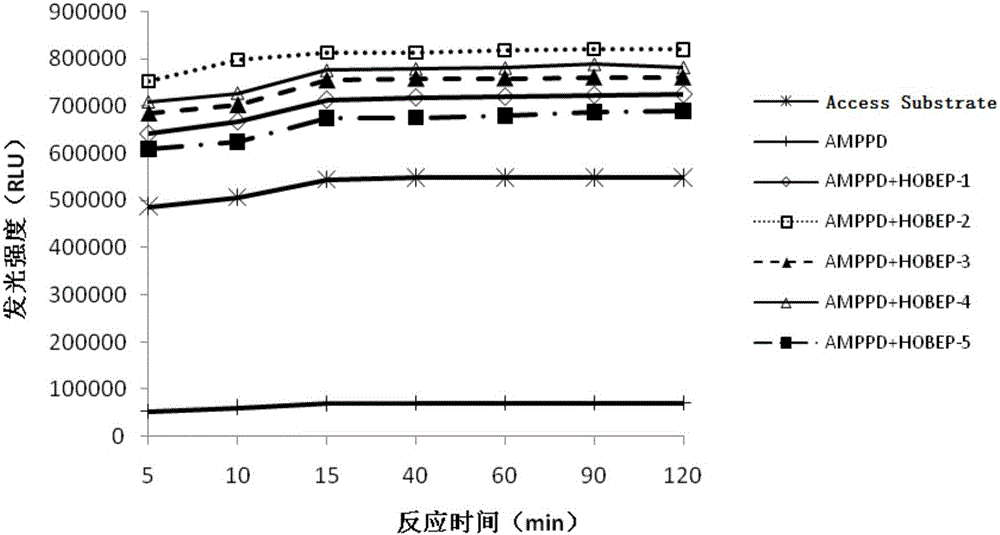
[0073] Example 2. Comparison of the enzymatic chemiluminescence substrate (APSH) and the Beckman chemiluminescence substrate (Access Substrate) in this example to test the AP signal intensity and linear relationship at each gradient concentration
[0074] The chemiluminescent substrate ASPH-1 prepared according to Table 1.2, the chemiluminescent substrate ASPH-2 prepared according to Table 2.2, the chemiluminescent substrate ASPH-3 prepared according to Table 3.2, and the chemiluminescent substrate prepared according to Table 4.2 were used respectively. The chemiluminescent substrate ASPH-4, the chemiluminescent substrate ASPH-5 prepared according to Table 5.2, and the commercially available chemiluminescent substrate Access Substrate of Beckman Company of the United States tested the signal intensity under the conditions of each gradient concentration AP (relative luminescence intensity RLU) and linear relationship, the test results are shown in Table 6 below.
[0075] Table ...
Embodiment 3
[0086] Embodiment three, compare enzymatic chemiluminescent substrate (APSH) of the present invention and Beckmann chemiluminescent substrate Access Substrate to measure the minimum detection limit (LOD) of thyroid-stimulating hormone (TSH) quantitative detection kit (magnetic particle chemiluminescence method) )
[0087] The chemiluminescent substrate ASPH-1 prepared according to Table 1.2, the chemiluminescent substrate ASPH-2 prepared according to Table 2.2, the chemiluminescent substrate ASPH-3 prepared according to Table 3.2, and the chemiluminescent substrate prepared according to Table 4.2 were used respectively. The minimum detection of chemiluminescent substrate ASPH-4, chemiluminescent substrate ASPH-5 prepared according to Table 5.2 and the commercially available chemiluminescent substrate Access Substrate from Beckman Company of the United States for the determination of thyroid-stimulating hormone (TSH) quantitative detection kit limit (LOD).
[0088] The content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com