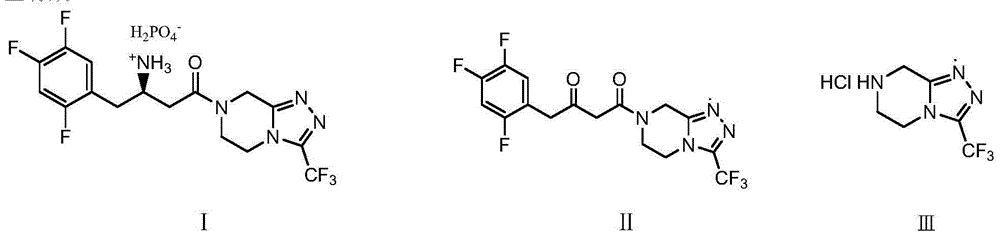
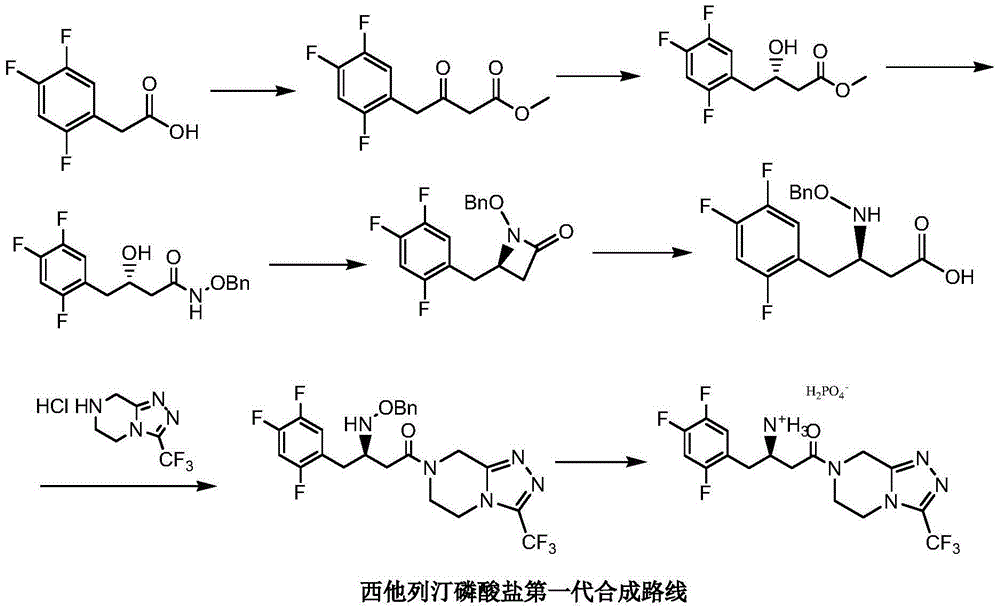
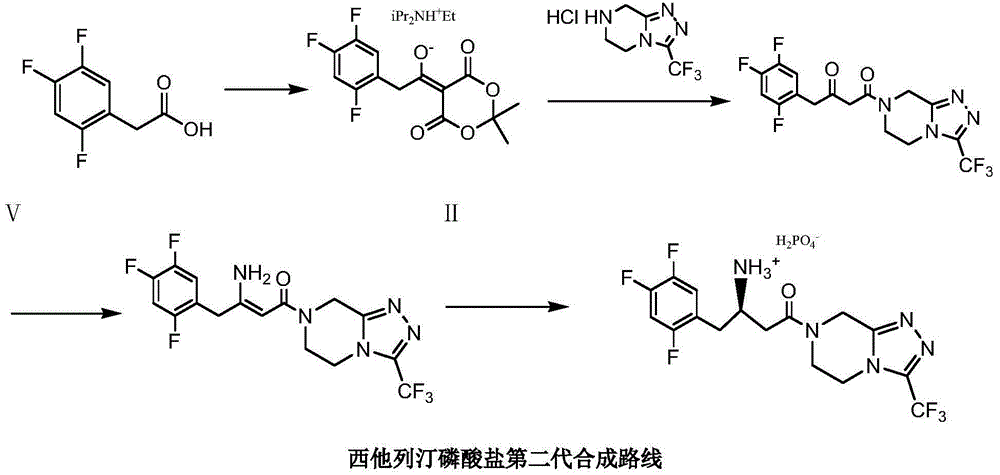
Low cost method for preparing sitagliptin phosphate salt key intermediate
A technology of sitagliptin and phosphate, applied in the direction of organic chemistry, etc., to achieve the effect of reducing the amount of waste water, low production cost and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H )yl]-1-(2,4,5-trifluorophenyl)-2-butanone (Ⅱ)
[0047] Step 1: 3-oxo-3-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H) Preparation of propionitrile (Ⅳ) toluene solution
[0048] Add 200 grams of toluene, 12 grams of sodium carbonate, 9.9 grams (0.1 moles) of methyl cyanoacetate, 22.8 grams (0.1 moles) of 5,6,7,8-tetrahydro-1,2 , 4-triazolo[4,3-a]pyrazine hydrochloride (Ⅲ), stirred and reacted at 92-95°C for 3 hours. Cool to 20-25°C, filter, and wash the filter cake (a mixture of sodium bicarbonate and sodium chloride) with 30 grams of toluene (washing a small amount of product adsorbed by the filter cake); combine the filtrates, and wash the combined filtrates with 5 grams of anhydrous Sodium sulfate was dried for 4 hours, filtered, and the filtrate (3-oxo-3-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a ] pyrazin-7 (8H) base] the toluene solution of pr...
Embodiment 2
[0053] Example 2: 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H )yl]-1-(2,4,5-trifluorophenyl)-2-butanone (Ⅱ)
[0054] Replace 9.9 grams (0.1 mole) of methyl cyanoacetate of embodiment 1 step 1 with 11.3 grams (0.1 moles) ethyl cyanoacetate, all the other are the same as embodiment 1, obtain 34.7 grams of white crystals 4-oxo-4-[ 3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)yl]-1-(2,4,5 -Trifluorophenyl)-2-butanone (II), the yield is 85.3% (calculated as III), and the liquid phase purity is 99.97%.
Embodiment 3
[0055] Example 3: 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H )yl]-1-(2,4,5-trifluorophenyl)-2-butanone (Ⅱ)
[0056] Replace 12 grams of sodium carbonate in Example 1 step 1 with 16.5 grams of potassium carbonate, and all the other are the same as in Example 1 to obtain 36.4 grams of white crystal 4-oxo-4-[3-(trifluoromethyl)-5,6-di Hydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)yl]-1-(2,4,5-trifluorophenyl)-2-butanone (Ⅱ) , the yield is 89.5% (calculated based on the compound of formula III), and the liquid phase purity is 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com