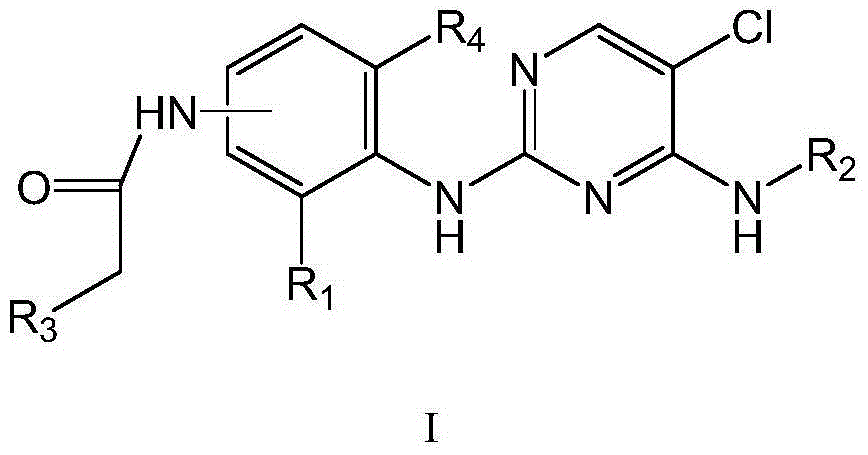
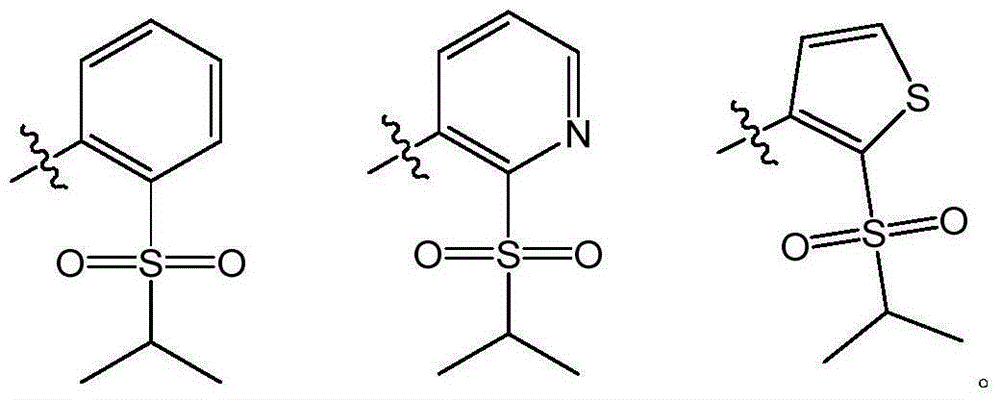
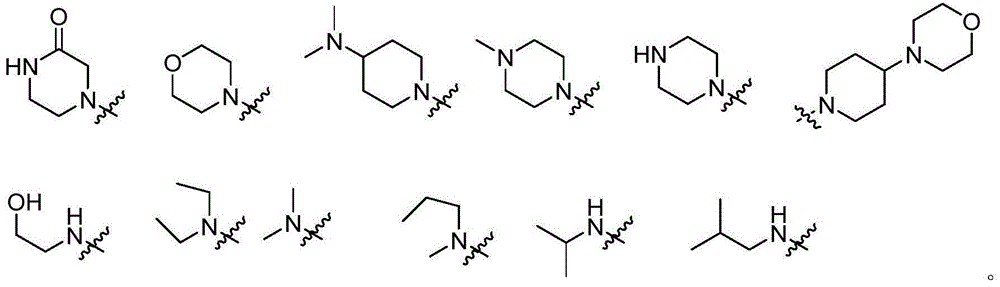
Pyrimidine derivative used as anaplastic lymphoma kinase (ALK) inhibitor
A solvate and compound technology, applied in the field of medicine, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1: Compound (intermediate) shown in preparation formula 2-1
[0108]
[0109] Put the compound shown in formula a (15g, 106.31mmol), potassium carbonate (29.38g, 212.61mmol) in a 500mL round bottom bottle, add 150ml of DMF, add isopropyl mercaptan (compound shown in formula b) under stirring (8.5g, 111.62mmol), the mixture was stirred and heated to 80°C, and reacted for 5 hours. After the reaction was completed, the reaction solvent was evaporated under reduced pressure, and the obtained crude product was washed with water, extracted with ethyl acetate, dried, concentrated, and obtained by column chromatography. The compound shown in c (yield 19.5 g, yield 95%).
[0110] The compound shown in formula c (19.5g, 98.86mmol) and m-chloroperoxybenzoic acid (mCPBA, the compound shown in formula d) (60.2g, 348.84mmol) were placed in a 1000mL round bottom bottle, and 500ml of dichloromethane was added , the mixture was stirred overnight at room temperature, quenc...
Embodiment 2
[0112] Embodiment 2: compound (intermediate) shown in preparation formula 2-2
[0113]
[0114] Compound shown in formula f (14.2g, 0.1mol), potassium carbonate (27.6g, 0.2mol) are placed in 500mL round bottom bottle, add 150 milliliters of DMF, add isopropyl thiol (compound shown in formula b) under stirring ) (8.0g, 0.105mol), the mixture was stirred and heated to 75°C, reacted for 6 hours, after the reaction was completed, the reaction solvent was evaporated under reduced pressure, the obtained crude product was washed with water, extracted with ethyl acetate, dried and concentrated, and obtained by column chromatography Compound represented by formula g (yield: 17.9 g, yield: 92.5%).
[0115] The compound shown in formula g (19.8g, 0.1mol) and m-chloroperoxybenzoic acid (mCPBA, the compound shown in formula d) (60.9g, 0.35mol) were placed in a 1000mL round bottom bottle, and 500ml of dichloromethane was added , the mixture was stirred overnight at room temperature, que...
Embodiment 3
[0117] Embodiment 3: compound (intermediate) shown in preparation formula 2-2
[0118]
[0119] Compound (15.9g, 0.1mmol) shown in formula j, potassium carbonate (27.6g, 0.2mmol) are placed in 500mL round bottom bottle, add 150 milliliters of DMF, add isopropyl mercaptan under stirring (compound shown in formula b ) (8.0g, 0.105mol), the mixture was stirred and heated to 80°C, reacted for 5 hours, after the reaction was completed, the reaction solvent was evaporated under reduced pressure, the obtained crude product was washed with water, extracted with ethyl acetate, dried and concentrated, and obtained by column chromatography Compound represented by formula g (yield: 18.1 g, yield: 93%).
[0120] The compound shown in formula g (19.8g, 0.1mol) and m-chloroperoxybenzoic acid (mCPBA, the compound shown in formula d) (48.7g, 0.28mol) were placed in a 1000mL round bottom bottle, and 500ml of dichloromethane was added , the mixture was stirred overnight at room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com