Full-automatic polypeptide extraction time-of-flight mass spectrometry detector
A mass spectrometer and spectral analysis technology, applied in the field of protein detection, can solve the problems of ineffective large-scale, time-consuming, and low throughput.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
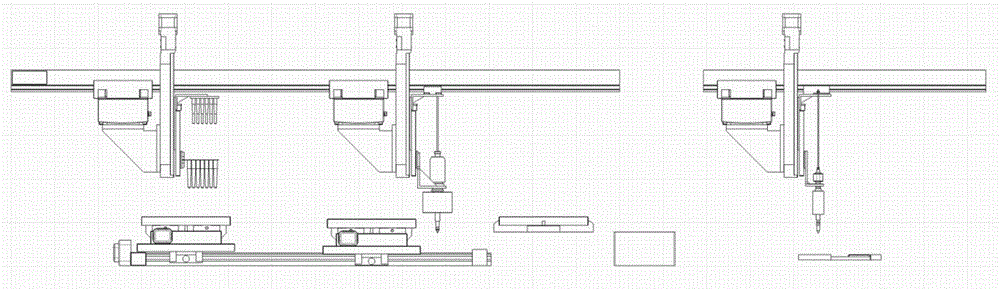
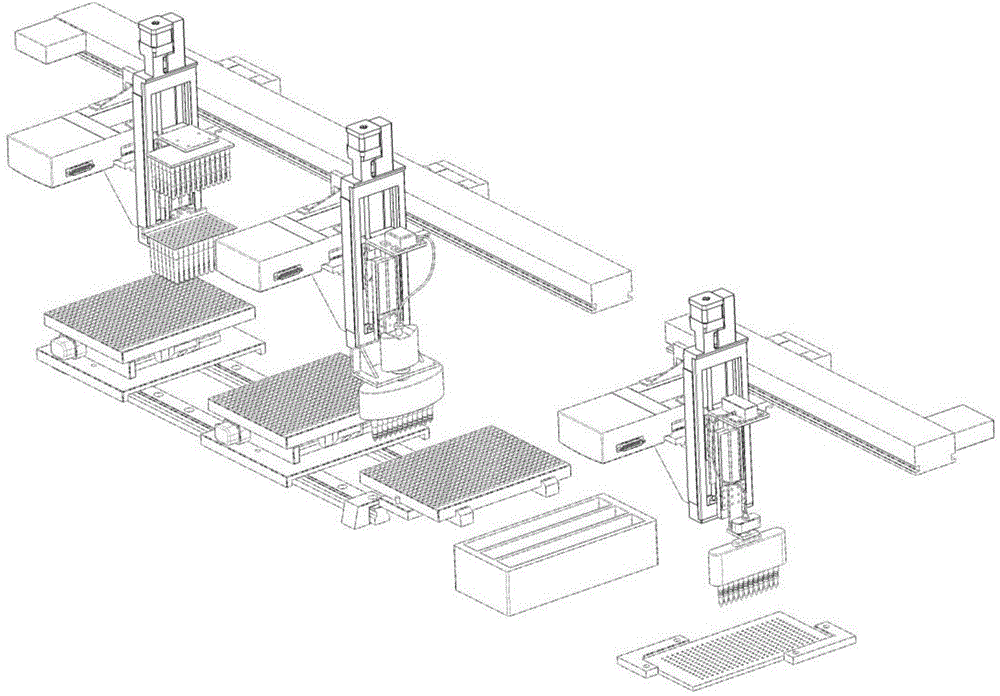
[0057] Embodiment one: the extraction of protein sample in body fluid and mobile distribution process (see Figure 4-6 )
[0058] 1. Add reagents and samples
[0059] In a 96-type deep well plate, add sample-magnetic beads-binding solution, sample-magnetic beads-binding solution, sample-magnetic beads-binding solution, washing solution, washing solution, washing solution , washing solution, washing solution, washing solution, eluting solution, eluting solution, eluting solution, and seal the 96-type deep-well plate with parafilm and place it in a moderate environment. Wherein the magnetic beads, binding solution, washing solution and eluent can all use reagents in known magnetic beads-protein extraction kits.
[0060] The sample plate to which the reagent has been added is fixed on the sample plate fixing table 110 according to the correct orientation.
[0061] 2. Setting method and parameters
[0062] Use an external computer to set up the method, take an operation name, ...
Embodiment 2
[0067] Embodiment two: sample packing
[0068] 1. Preparation
[0069] Put the sample to be divided into the sample collecting plate 206, install it on the sample plate fixing table 110, and move to the position of the right locking slot 113 driven by the stepping motor 112. Fix the sample collection plate 206 in the corresponding position.
[0070] 2. Method parameter setting
[0071] In the external computer program, take an operation name, such as "sample dispensing", and then set the well position of the required dispensing sample and the corresponding well position in the sample collection plate, dispensing volume, pipetting speed and other parameters. After setting all the parameters, save the method and select the method in the operation.
[0072] 3. Packing samples
[0073] Select the corresponding method and start dispensing samples. Under the control of the external computer, the pipette 204 moves to the top of the cleaning device 32, and is successively cleaned...
Embodiment 3
[0074] Embodiment three: Chip sample spotting (referring to Figure 7-9 )
[0075] Under the control of an external computer program, the sample pointing device first cleans the sample point needle 306 . The spotting instrument 3 or the spotting device 3 moves to the top of the cleaning device 32, successively washes through 100% alcohol, 50% alcohol and ultrapure water, then dries in the drying chamber 325, and then moves to the top of the sample collection plate 206 , accurately absorb the extraction solution and its volume, then move to the top of the chip 307, and accurately point the extraction solution on the set target position. Complete a spotting cycle. In this way, all the extracts that need to be spotted are spotted on the corresponding target positions. Let it dry naturally at room temperature, and then apply the matrix.
[0076] The prepared matrix solution is added to the matrix plate, the sample collection plate 206 is removed, and the matrix plate is fixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com