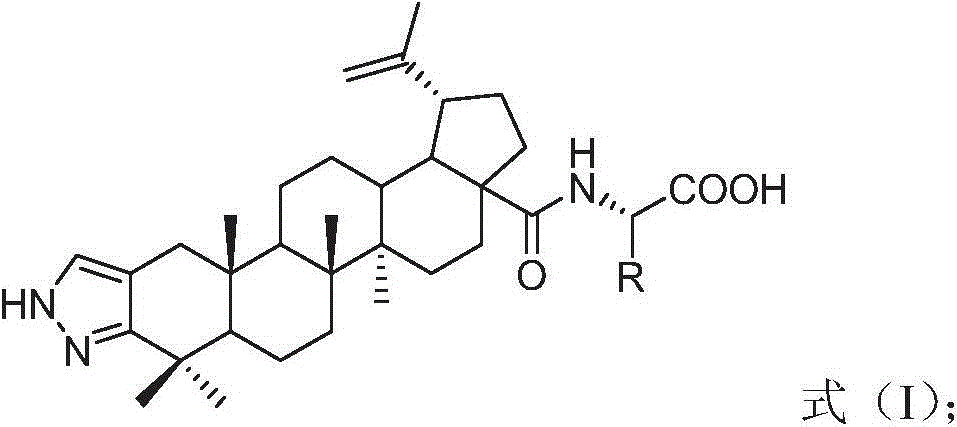
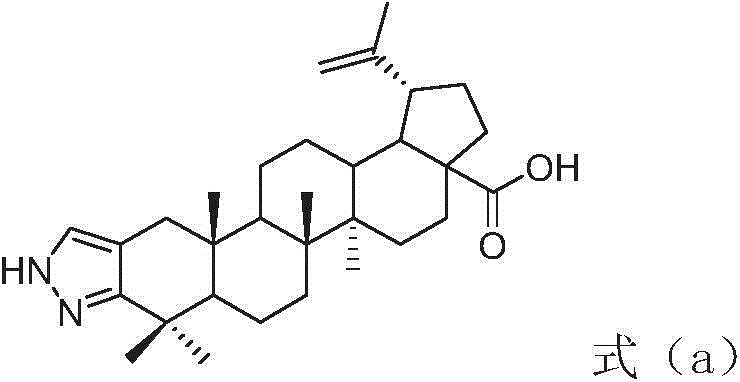
Betulinic acid-amino acid derivative, and preparation method and application thereof
A technology of betulinic acid and amino acids, which is applied in the field of medicine and its preparation and application, can solve the problems of seriousness, side effects, and increased risk of osteosarcoma, and achieve the effect of enhanced activity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of betulinic acid-amino acid derivative shown in formula (I) of the present invention comprises the following steps:
[0030] Step 1, using compound a (XJ-479) as a raw material to acylate with 1.5 times the molar amount of acetic anhydride and 1 times the molar amount of pyridine under nitrogen protection for 0.5 hours to obtain compound b, the solvent is anhydrous tetrahydrofuran, and the reaction temperature is 30 °C, the obtained product was purified by silica gel column chromatography to obtain betulinic acid derivative b with a yield of 87%.
[0031] In step 2, compound b was reacted with 5 times molar amount of oxalyl chloride in dichloromethane under the protection of nitrogen for 18 hours to obtain compound c, which was directly used in the next step without purification.
[0032] Step 3, compound c is mixed with 1.2 times the molar amount of glycine methyl ester hydrochloride, L-alanine methyl ester hydrochloride, L-valine methyl ester h...
Embodiment 1
[0035] Embodiment 1: Preparation of betulinic acid derivative CL-1
[0036]
[0037] Under the protection of nitrogen, 480 mg of compound a was dissolved in a mixed solvent of 10 mL THF, 1.5 times the molar amount of acetic anhydride and 1 times the molar amount of pyridine were added dropwise, the reaction temperature was 30°C, and the reaction was stirred for 0.5 hours. After the reaction was completed, 50 mL of ethyl acetate and 10 mL of saturated aqueous sodium bicarbonate were added, the aqueous layer was extracted twice with 100 mL of ethyl acetate, the organic layers were combined, washed with saturated brine, anhydrous Na 2 SO 4 The crude product obtained by drying and concentrating under reduced pressure was subjected to silica gel column chromatography (PE:EA=2:1) to obtain 302 mg of compound b with a yield of 87%. 1 HNMR (CDCl 3 , 400MHz) δ: 7.94(s, 1H), 4.70(s, 1H), 4.57(s, 1H), 3.00-2.94(m, 1H), 2.53-2.50(m, 1H), 2.31-2.26(m, 1H), 2.14-2.12(m, 1H), 2.01(s,...
Embodiment 2
[0041] Embodiment 2: the preparation of betulinic acid derivative CL-2
[0042] In this example, steps 1, 2, and 3 are the same as in Example 1, that is, compound d(R'=CH 3 ) The synthetic method is similar to embodiment 1, just changes raw material glycine methyl ester hydrochloride into L-alanine methyl ester hydrochloride. Yield 65%. 1 H NMR (400MHz, DMSO) δ7.99(s, 1H), 7.41(d, J=7.4Hz, 1H), 7.19(s, 1H), 6.92(s, 1H), 4.67(s, 1H), 4.55 (s, 1H), 4.19(m, 3H), 2.99(td, J=10.9, 4.2Hz, 2H), 2.60(m, 2H), 2.51(s, 3H), 2.17(d, J=13.0Hz, 1H), 2.07-1.83(m, 3H), 1.77(m, 1H), 1.65(s, 3H), 1.60-1.33(m, 11H), 1.31(s, 3H), 1.30(s, 3H), 1.28 (s, 3H), 1.26(s, 3H), 1.22(s, 2H), 1.20(s, 3H), 1.17-0.98(m, 3H), 0.96(s, 3H), 0.90(s, 3H), 0.71(s, 3H).
[0043] Dissolve 300 mg of compound d in 10 mL of THF, add 0.2 mL of distilled water, add 3 times the molar amount of LiOH, stir at room temperature for two hours, wash twice with 1M HCl after the reaction, wash with 10 mL of saturated saline, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com