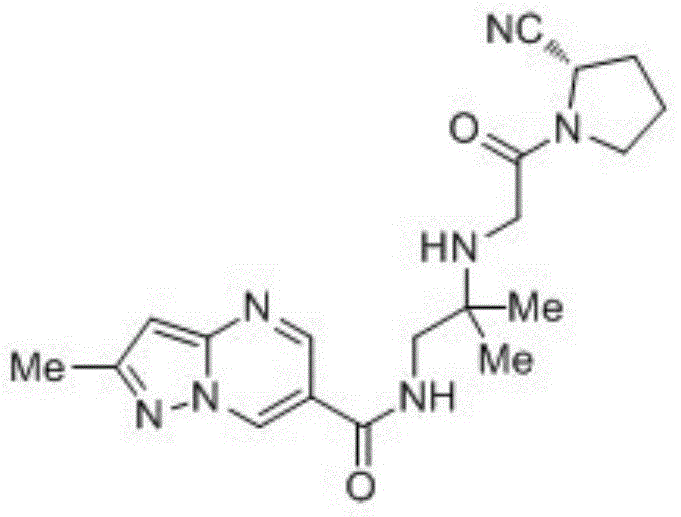
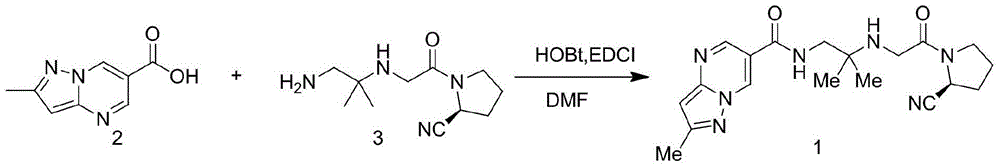
Preparation method of anagliptin
The technology of a compound and a basic substance is applied in the field of preparing an antidiabetic drug Analiptin, which can solve the problems of expensive hydroxybenzotriazole condensing agent, difficult industrial scale-up, harsh reaction conditions, etc., and achieves great practical application value, The effect of high overall yield and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
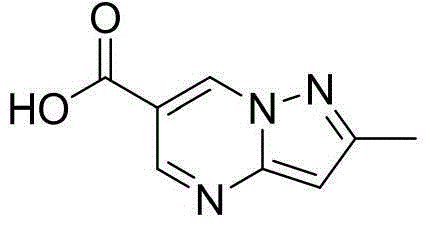
[0075] O-2,5-dicarbonylpyrrolyl-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate (5-1)
[0076] Suspend the compound of formula (1) (30g) and N-hydroxysuccinimide (19.5g) in 200mL of dichloromethane, add EDCI (36g) in dichloromethane mixture in batches under ice-cooling, and stir at room temperature after adding 8 hours. After filtration, 40 g of white solid O-2,5-dicarbonylpyrrolyl-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate (5-1) was obtained, with a yield of 86%.
[0077] 1 HNMR (DMSO-d6): δ1.90-2.05 (m, 4H), 2.51 (s, 3H), 6.66 (s, 1H), 8.81 (s, 2H), 9.36 (s, 1H).
Embodiment 2
[0079] O-3,5-dimethoxy-2,4,6-triazinyl-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate (5-2)
[0080] According to General Method 1, triethylamine was used to prepare (5-2) white solid in dichloromethane, and the yield was 95%.
[0081] 1 HNMR (DMSO-d6): δ2.51 (s, 3H), 3.90 (s, 6H), 6.66 (s, 1H), 8.81 (s, 2H), 9.36 (s, 1H).
Embodiment 3
[0083] O-3-Chloro-5-methoxy-2,4,6-triazinyl-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxylate (5-3)
[0084] According to General Method 1, (5-3) was prepared as a white solid with pyridine in THF, and the yield was 84%.
[0085] 1 HNMR (DMSO-d6): δ2.55 (s, 3H), 3.91 (s, 3H), 6.68 (s, 1H), 8.71 (s, 2H), 9.46 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com