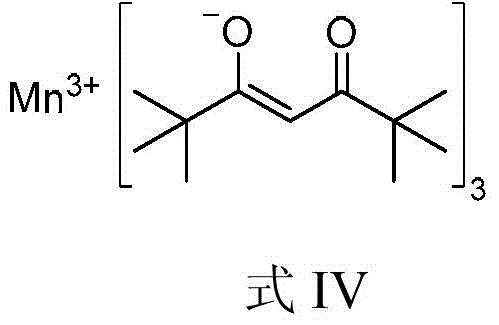
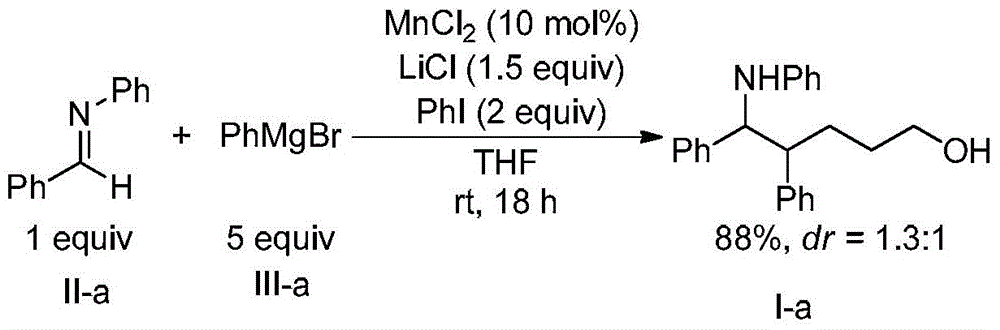
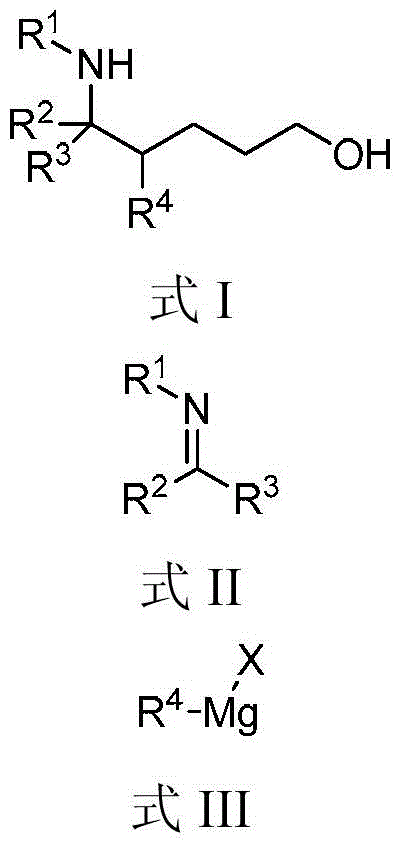A kind of method for preparing 1,5-aminoalcohol
A technology of amino alcohol and phenyl, which is applied in the field of preparation 1, can solve the problems of cumbersome synthesis of raw materials, harsh experimental conditions, and expensive reagents, and achieve the effects of cheap and easy-to-obtain reagents, mild reaction conditions, and great industrialization potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, 4,5-diphenyl-5-(phenylamino)-1-pentanol (formula Ⅰ-a)
[0026]
[0027] Add N-benzylideneaniline (Formula II-a) (2mmol, 362mg), catalyst manganese dichloride (0.2mmol, 25mg), additive lithium chloride (3mmmol, 127mg), oxidant iodobenzene ( 4mmol, 816mg), solvent tetrahydrofuran (5mL) and phenylmagnesium bromide (formula III-a) (tetrahydrofuran solution, 1mol / L) (10mmol, 10mL), three-component reaction at room temperature. After 18h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-a) in the crude product was detected by H NMR to be 1.3:1. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 583 mg of the target product (Formula I-a), with a yield of 88%.
[0028] The major d...
Embodiment 2
[0030] Example 2, 4-phenyl-5-(methylthiophenyl)-5-(phenylamino)-1-pentanol (formula Ⅰ-b)
[0031]
[0032] Add imine (formula II-b) (2mmol, 454mg), catalyst manganese diacetate (0.1mmol, 17mg), lithium chloride (3mmmol, 127mg), iodobenzene (3mmol, 612mg), tetrahydrofuran ( 9.3mL) and phenylmagnesium chloride (formula III-b) (tetrahydrofuran solution, 1.4mol / L) (8mmol, 5.7mL) at 0°C for a three-component reaction. After 24 h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-b) in the crude product was 1.4:1 as detected by H NMR. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 718 mg of the target product (Formula I-b), with a yield of 95%.
[0033] The major diastereomers are characterize...
Embodiment 3
[0035] Example 3, 4-phenyl-5-(4-chlorophenyl)-5-((2,4,6-trimethylphenyl)amino)-1-pentanol (Formula I-c)
[0036]
[0037] Add imine (Formula II-c) (2mmol, 516mg), catalyst manganese diacetylacetonate (0.1mmol, 25mg), lithium chloride (3mmmol, 127mg), iodobenzene (3mmol, 612mg), tetrahydrofuran into a 50mL flask in sequence (9.3mL) and phenylmagnesium chloride (formula III-b) (tetrahydrofuran solution, 1.4mol / L) (8mmol, 5.7mL) at 40°C for a three-component reaction. After 18h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-c) in the crude product was determined to be 3.3:1 by H NMR. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 775 mg of the target product (Formula I-c), with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com