Preparation method of high-efficiency nickel-based catalyst for producing hydrogen in methanol-steam reforming
A technology for producing hydrogen from methane steam and reforming, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. The preparation method is simple, the activity is stable, and the conditions are easy to control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Ni / Y 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0025] (1) Y(NO) with Y:Zr (molar ratio) of 1:1 3 ) 3 ·6H 2 O, Zr(NO 3 ) 4 ·5H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
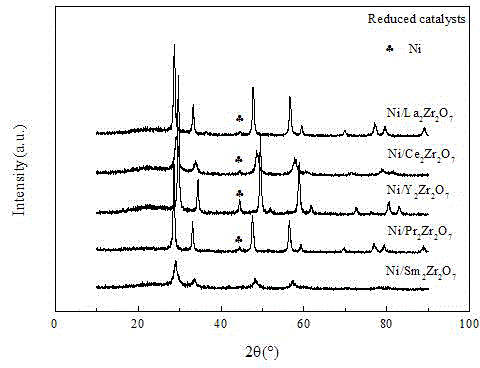
[0026] (2) Dilute industrial ammonia water with a concentration of 25% to 1 time as a precipitant, and slowly add ammonia water dropwise to the above solution (1) to pH = 2-3 under continuous stirring at 40 ° C, and after stirring for 1 hour, at 80 Evaporate in an oven at ℃ to form a gel, rise to the ignition temperature of 200℃ until the combustion reaction occurs; dry the combustion product at 110℃ for 12 hours, and bake in a high-temperature furnace at 800℃ for 4 hours. The furnace temperature setting program is: room temperature at 2℃ / min to the target temperature of 800°C. Produced rare earth Y 2 Zr 2 o 7 Pyrochlore composite oxide support, characterized by XRD, such as figure 1 As shown, the carrier h...
Embodiment 2
[0030] Ni / La 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0031] (1) La(NO 3 ) 3 ·6H 2 O, Zr(NO 3 ) 4 ·5H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
[0032] (2) Dilute industrial ammonia water with a concentration of 25% to 1 time as a precipitant, and slowly add ammonia water dropwise to the above solution (1) to pH = 2-3 under continuous stirring at 40 ° C, and after stirring for 1 hour, at 80 Evaporate in an oven at ℃ to form a gel, rise to the ignition temperature of 300℃ until the combustion reaction occurs; dry the combustion product at 110℃ for 12 hours, and bake in a high-temperature furnace at 800℃ for 4 hours. The furnace temperature setting program is: room temperature at 2℃ / min to reach the target temperature. Rare earth La 2 Zr 2 o 7 Pyrochlore composite oxide support, characterized by XRD, such as figure 1 As shown, the carrier has pyrochlore-type structure characterist...
Embodiment 3
[0036] Ni / Sm 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0037] (1) Sm(NO 3 ) 3 ·6H 2 O, Zr(NO 3 ) 4 ·5H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
[0038] (2) Dilute industrial ammonia water with a concentration of 25% to 1 time as a precipitant, and slowly add ammonia water dropwise to the above solution (1) to pH = 2-3 under continuous stirring at 40 ° C, and after stirring for 1 hour, at 80 Evaporate in an oven at ℃ to form a gel, rise to the ignition temperature of 300℃ until the combustion reaction occurs; dry the combustion product at 110℃ for 12 hours, and bake in a high-temperature furnace at 800℃ for 4 hours. The furnace temperature setting program is: room temperature at 2℃ / min to reach the target temperature. Produced rare earth Y 2 Zr 2 o 7 Pyrochlore composite oxide support, characterized by XRD, such as figure 1 As shown, the carrier has pyrochlore-type structure char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com