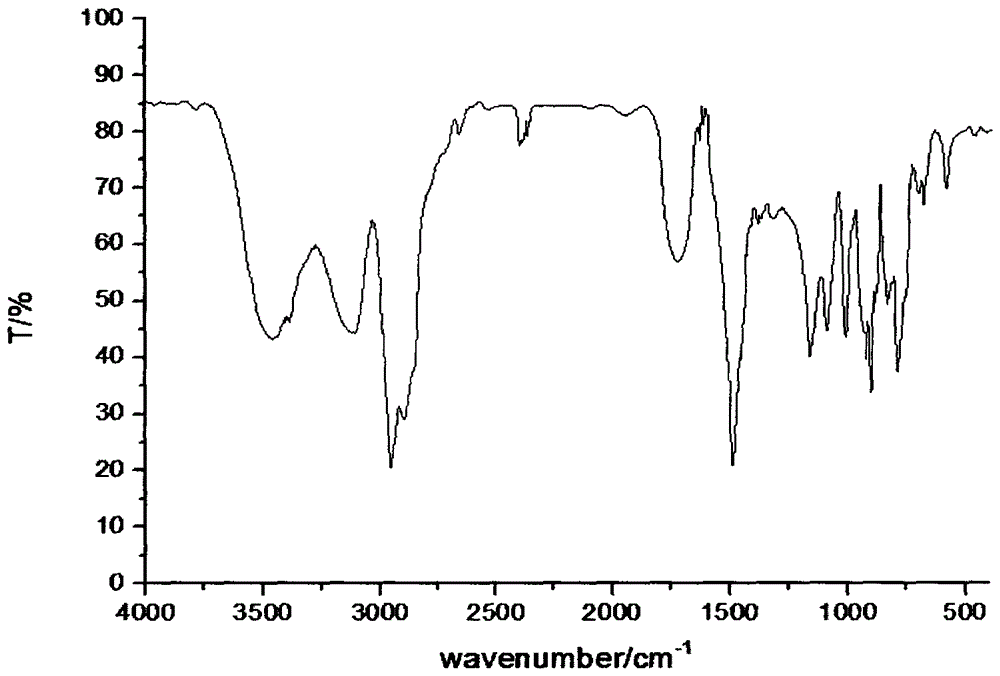
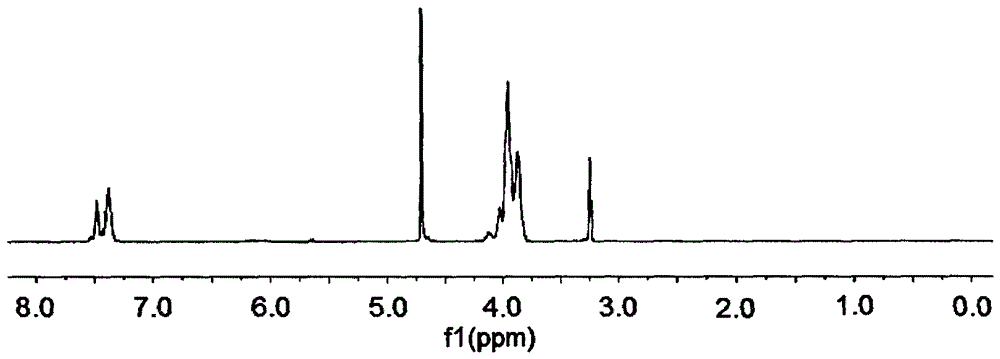
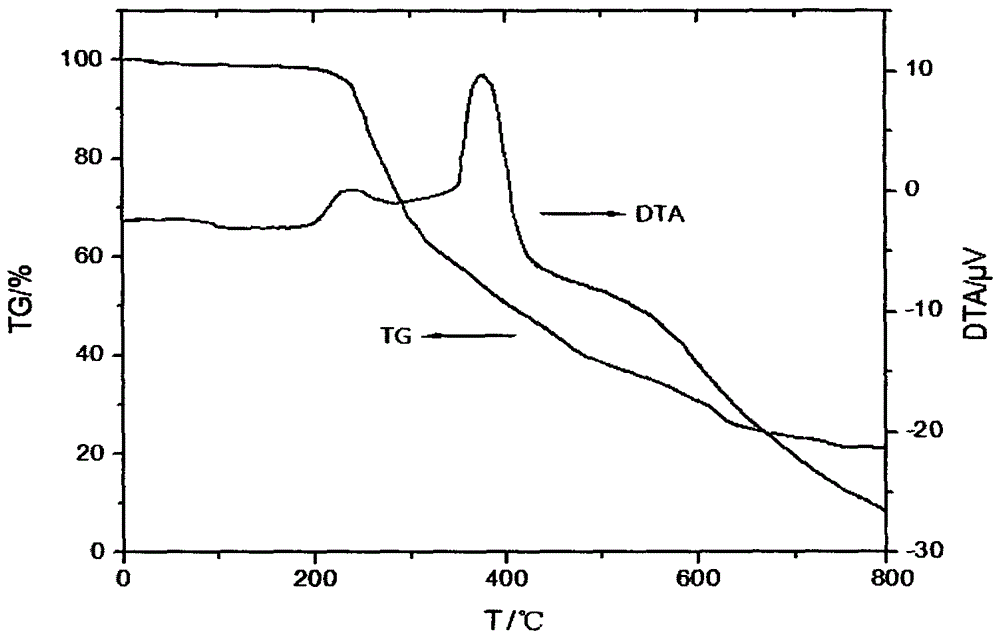
Hydroxymethylphosphonothioheterocyclic phenylthiophosphonate compound and preparation method thereof
A technology of phenylphosphonothioate and hydroxymethylphosphonothioate, which is applied in the field of hydroxymethylphosphonothiophosphono heterocyclic phenylthiophosphonate compounds and their preparation, and can solve problems such as hazards , to achieve the effects of simple process, high performance of phosphorus and sulfur synergistic flame retardant, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 In a 100ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and connected to a hydrogen chloride absorbing device on the condenser, the air in the bottle was driven away with nitrogen, and 15.60g (0.1mol) of three Hydroxymethylphosphine sulfide, under stirring, at 20°C, add 21.10g (0.1mol) phenylphosphonothiodichloride dropwise, make it fully mixed, heat up to 80°C, hydrogen chloride begins to be released, heat up to 110°C Insulate for 3 hours, then raise the temperature to 160°C for 9 hours, wait until no hydrogen chloride is released, then vacuum the hydrogen chloride to exhaust, cool down to below 10°C, add 50ml of ice water, stir, and add 5% sodium carbonate solution dropwise to make the reaction system pH = 7, standing for stratification, separating the lower layer material liquid, and removing a small amount of water in the material by distillation under reduced pressure to obtain the product hydroxymethylphosph...
Embodiment 2
[0030] Example 2 In a 100ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and connected to a hydrogen chloride absorbing device on the condenser, the air in the bottle was driven away with nitrogen, and 15.60g (0.1mol) of three Hydroxymethylphosphine sulfide, under stirring, at 20°C, add 21.10g (0.1mol) phenylphosphonothiodichloride dropwise, make it fully mixed, heat up to 80°C, hydrogen chloride begins to be released, heat up to 110°C Keep warm for 3 hours, then raise the temperature to 130°C and keep it warm for 12 hours, wait until no hydrogen chloride is released, then vacuum the hydrogen chloride to exhaust, lower the temperature to below 10°C, add 40ml of ice water, add 5% sodium carbonate solution dropwise under stirring, and make the reaction system pH = 7, standing for stratification, separating the lower layer material liquid, and removing a small amount of water in the material by distillation under reduced pressure to ...
Embodiment 3
[0031]Example 3 In a 150ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and connected to a hydrogen chloride absorption device on the condenser, the air in the bottle was driven away with nitrogen, and 50ml xylene was added, and 15.60g ( 0.1mol) trimethylol phosphine sulfide, under stirring, at 20°C, add dropwise 21.10g (0.1mol) phenylphosphonothioate dichloride, make it fully mixed, heat up to 80°C, hydrogen chloride begins to be released, Raise the temperature to 110°C for 3 hours, then raise the temperature to 140°C for 9 hours, wait for no hydrogen chloride to be released, remove xylene (recycled) by distillation under reduced pressure, cool down to below 10°C, add 40ml of ice water, stir, and drop 5 % sodium carbonate solution to make the pH of the reaction system=7, let it stand for stratification, separate the lower layer material liquid, and distill under reduced pressure to remove a small amount of water in the material t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com