Acrylic acid catalyst and synthesis method of acrylic acid
A catalyst, acrylic technology, used in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of poor stability and low selectivity, and achieve molding handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
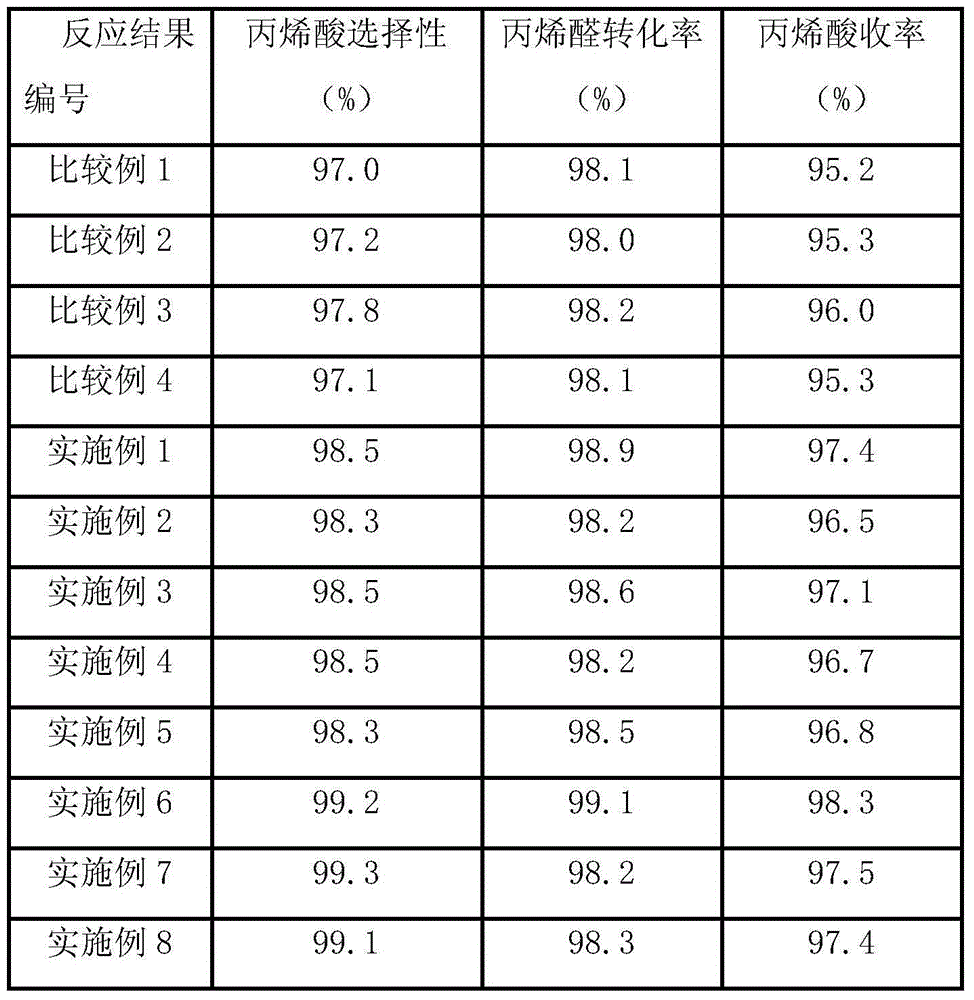
Examples
Embodiment 1
[0084] 1. Catalyst preparation
[0085] a) 500 grams of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O and 13.3 grams of KOH were added to 500 grams of warm water at 70°C, and stirred to make them all dissolve to form material I;
[0086] b) Add 123.5 grams of ammonium metavanadate NH to 250 grams of water 4 VO 3 forming material II;
[0087] c) 52.1 grams of antimony trioxide Sb 2 o 3 Add 100 grams of 70 ℃ hot water, stir and dissolve, then add 171 grams of copper nitrate Cu(NO 3 ) 2 ·3H 2 O, 82 grams of lanthanum nitrate La (NO 3 ) 2 .6H 2 O, 82 grams of cerium nitrate Ce (NO 3 ) 2 .6H 2 O and 95.3 grams of ammonium tungstate stirring and dissolving make material III;
[0088] d) adding materials II and III dropwise to material I successively under rapid stirring to form a catalyst slurry, and stirring and aging at 100° C. for 3 hours;
[0089] e) the slurry is spray-dried to obtain the catalyst precursor 1;
[0090] f) pre-calcining the catalyst prec...
Embodiment 2
[0096] 1. Catalyst preparation
[0097] a) 500 grams of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O and 13.3 grams of KOH were added to 500 grams of warm water at 70°C, and stirred to make them all dissolve to form material I;
[0098] b) Add 123.5 grams of ammonium metavanadate NH to 250 grams of water 4 VO 3 forming material II;
[0099] c) 52.1 grams of antimony trioxide Sb 2 o 3 Add 100 grams of 70 ℃ hot water, stir and dissolve, then add 171 grams of copper nitrate Cu(NO 3 ) 2 ·3H 2 O, 82 grams of lanthanum nitrate La (NO 3 ) 2 .6H 2 O, 82 grams of praseodymium nitrate Pr (NO 3 ) 2 .6H 2 O and 95.3 grams of ammonium tungstate stirring and dissolving make material III;
[0100] d) adding materials II and III dropwise to material I successively under rapid stirring to form a catalyst slurry, and stirring and aging at 100° C. for 3 hours;
[0101] e) the slurry is spray-dried to obtain the catalyst precursor 1;
[0102] f) pre-calcining the catalys...
Embodiment 3
[0108] 1. Catalyst preparation
[0109] a) 500 grams of ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O and 13.3 grams of KOH were added to 500 grams of warm water at 70°C, and stirred to make them all dissolve to form material I;
[0110] b) Add 123.5 grams of ammonium metavanadate NH to 250 grams of water 4 VO 3 forming material II;
[0111] c) 52.1 grams of antimony trioxide Sb 2 o 3 Add 100 grams of 70 ℃ hot water, stir and dissolve, then add 171 grams of copper nitrate Cu(NO 3 ) 2 ·3H 2 O, 82 grams of praseodymium nitrate Pr (NO 3 ) 2 .6H 2 O, 82 grams of cerium nitrate Ce (NO 3 ) 2 .6H 2 O and 95.3 grams of ammonium tungstate stirring and dissolving make material III;
[0112] d) adding materials II and III dropwise to material I successively under rapid stirring to form a catalyst slurry, and stirring and aging at 100° C. for 3 hours;
[0113] e) the slurry is spray-dried to obtain the catalyst precursor 1;
[0114] f) pre-calcining the catalyst pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com