Precious metal loaded TiO2 nanorod photocatalyst preparation method
A photocatalyst, precious metal technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of complex preparation steps and high production costs, and achieve the preparation process Simple, low cost and high photocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Dissolve titanium sulfate, silver nitrate, hexamethylenetetramine, and sodium hydroxide in water and stir evenly to obtain a mixed solution with a concentration of 0.025mol L of titanium sulfate -1 , silver nitrate 1.0% (molar ratio to titanium sulfate), hexamethylenetetramine 0.025mol L -1 , sodium hydroxide 10mol L -1 ;
[0018] 2. Transfer the above mixed solution to a high-pressure reactor lined with tetrafluoroethylene, and heat at a constant temperature of 180°C for 24 hours;
[0019] 3. After cooling the above reaction mixture, centrifuge, pour off the upper liquid, soak the obtained precipitate in 0.02mol / L sulfuric acid solution for 2h, centrifuge, and wash with water to obtain a silver-loaded precipitate;
[0020] 4. Put the precipitate obtained in step (3) into a drying oven at 60°C and dry it, then bake it at 700°C for 0.5h to obtain silver-loaded TiO 2 Nano stave.
Embodiment 2
[0022] 1. Dissolve titanium sulfate, chloroplatinic acid, hexamethylenetetramine, and sodium hydroxide in water and stir evenly to obtain a mixed solution with a concentration of 0.05mol L of titanium sulfate -1 , chloroplatinic acid 0.05%, hexamethylenetetramine 0.05mol L -1 , sodium hydroxide 8mol L -1 ;
[0023] 2. Transfer the above mixed solution to a high-pressure reactor lined with tetrafluoroethylene, and heat at a constant temperature of 180°C for 18 hours;
[0024] 3. After cooling the above reaction mixture, centrifuge, pour off the upper liquid, soak the obtained precipitate in 0.02mol / L sulfuric acid solution for 1h, centrifuge, and wash with water to obtain a platinum-loaded precipitate;
[0025] 4. Put the precipitate obtained in step (3) into a drying oven at 60°C and dry it, then bake it at 600°C for 1 hour to obtain platinum-supported TiO 2 Nano stave.
Embodiment 3
[0027] 1. Dissolve titanium sulfate, palladium chloride, hexamethylenetetramine, and sodium hydroxide in water and stir evenly to obtain a mixed solution with a concentration of 0.1mol L of titanium sulfate -1 , palladium chloride 0.1%, hexamethylenetetramine 0.1mol L -1 , sodium hydroxide 12mol L -1 ;
[0028] 2. Transfer the above mixed solution to a high-pressure reactor lined with tetrafluoroethylene, and heat at a constant temperature of 200°C for 8 hours;
[0029] 3. After cooling the above reaction mixture, centrifuge, pour off the upper liquid, soak the obtained precipitate in 0.04mol / L sulfuric acid solution for 1h, centrifuge, and wash with water to obtain a palladium-loaded precipitate;
[0030] 4. Put the precipitate obtained in step (3) into a 60°C drying oven for drying, and then bake it at 600°C for 1 hour to obtain palladium-supported TiO 2 Nano stave.
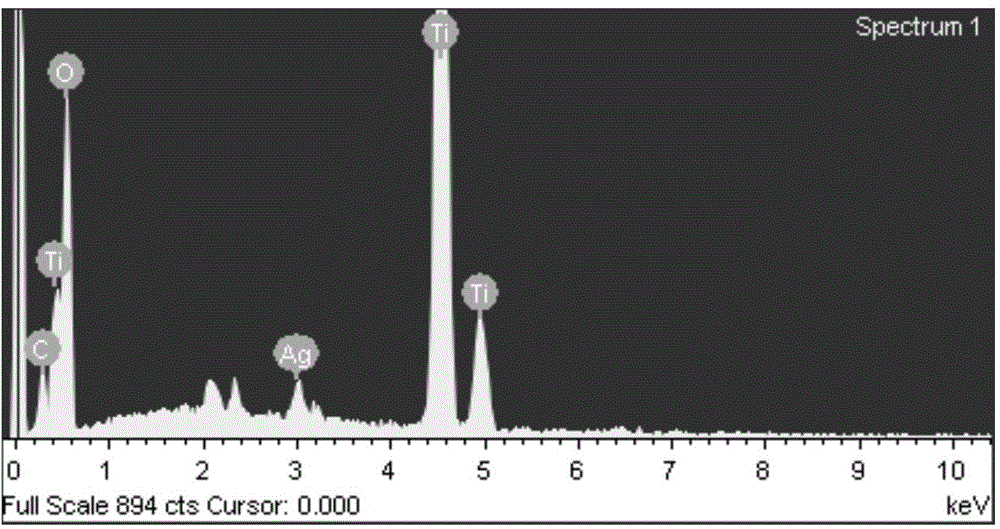
[0031] The energy spectrum analysis was carried out on the energy spectrometer attached to the JSM-6700F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com