A kind of preparation method and application of high molecular weight deacetylated hyaluronic acid
A molecular weight, high-efficiency liquid phase technology, applied in the field of polysaccharide modification, can solve the problems of glycosidic bond breakage, hydrazine hydrate deacetylation effect is not very ideal, high molecular weight products cannot be obtained, etc., to achieve the effect of easy operation and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Preparation of high molecular weight deacetylated hyaluronic acid
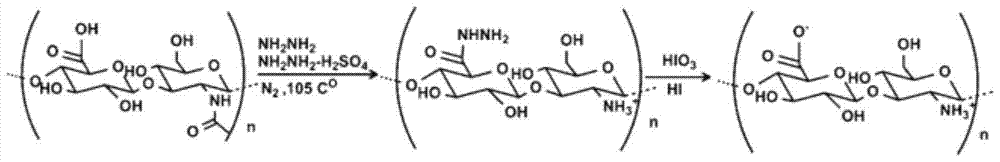
[0031] like figure 1 Shown, a kind of preparation method of high molecular weight deacetylated polysaccharide specifically comprises the following steps:
[0032] 1) Preparation of anhydrous hydrazine: Add 12 grams of potassium hydroxide to 30 mL of hydrazine monohydrate, overnight at 4°C, take the supernatant, add 12 grams of sodium hydroxide, protect with nitrogen, heat and reflux for 2 hours, change to a distillation device, and collect the distillate , to obtain 90-94% anhydrous hydrazine.
[0033] 2) Dissolving hyaluronic acid with anhydrous hydrazine containing hydrazine sulfate at a weight concentration of 1%, reacting at 60° C. for 72 hours after deoxygenated nitrogen protection;
[0034] 3) The reaction product is precipitated with cold ethanol, and the precipitate is dissolved in glacial acetic acid with a concentration of 5%. Add iodic acid with a concentration of 5g / L, and pla...
Embodiment 2
[0037] Example 2 Proliferation of polysaccharide spleen lymphocytes
[0038] 1) Acquisition of spleen lymphocytes: Take 5 B6 mice, kill them with nitrogen gas, soak them in 70% ethanol for 10 minutes, open the abdominal cavity of the mice under aseptic conditions to take the spleen, grind them through a 70-micron sieve with the inner core of a syringe, and pour them into PBS Flush with liquid, pass through a 40-micron sieve, collect the separated splenocyte suspension and divide it into five tubes, centrifuge at 1000r / min for 5min, and discard the supernatant. Add 12mL of ammonium chloride erythrocyte lysate to each centrifuge tube, mix the spleen cells, let stand for 5-6min, wait for the erythrocytes to be completely broken, centrifuge at 1000r / min for 5min, discard the supernatant to remove the erythrocytes, wash 1-2 times with PBS, and use RPMI -1640 (containing 10% fetal bovine serum) resuspended cells, counted to adjust the cell concentration to 8~10×10 6 a / mL;
[0039]...
Embodiment 3
[0042] Example 3 Spleen Cell Proliferation Experiment Using TLR2- / - and TLR4- / - Deficient Mice
[0043] 1) The acquisition of splenic lymphocytes was the same as in Example 2, and the spleen cells of TLR2- / - and TLR4- / - and their control mice B6 and B10 were obtained respectively;
[0044] 2) Drug-stimulated splenocyte proliferation experiment: the same as in Example 2, plating, adding drugs, culturing, and detecting;
[0045] 3) Calculate the proliferation rate according to the absorbance graph, and compare whether the proliferation effect of the drug on splenocytes depends on the TLR2 or TLR4 pathway, such as Figure 5 As shown, the results showed that the proliferation of splenic lymphocytes by HA-72 was dependent on the TLR4 pathway, whereas the proliferation of splenic lymphocytes by dHA-72 was dependent on the TLR2 pathway.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com