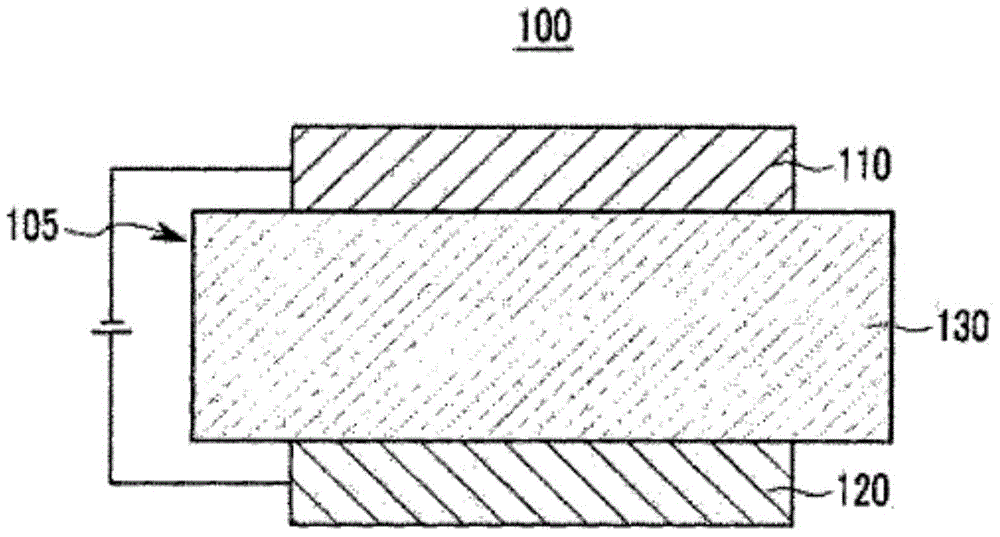
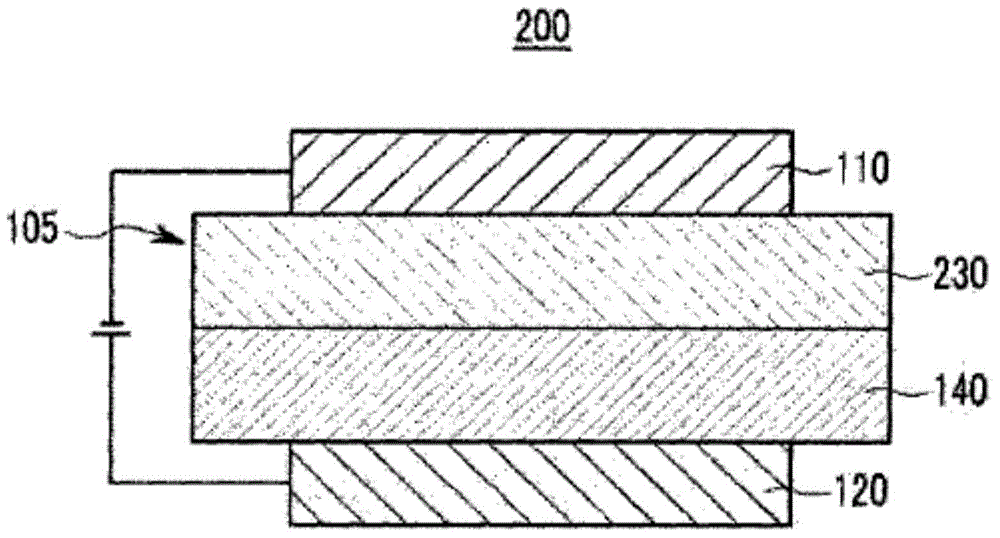
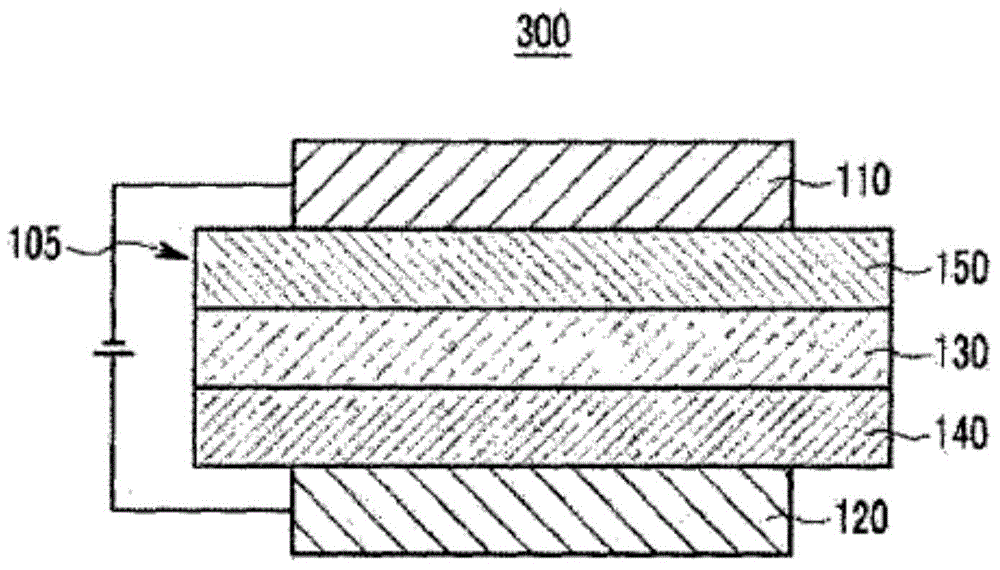
Organic optoelectronic device, and display device including same
An optoelectronic device, organic technology, applied in photovoltaic power generation, circuits, luminescent materials, etc., can solve problems such as unsatisfactory development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1288] Example 1: Preparation of Compound F-94
[1289]
[1290] In a round bottom flask, 10 g (30.9 mmol) of intermediate M-1, 9.9 g (30.9 mmol) of bis(4-benzidine)amine and 4.5 g (46.35 mmol) of sodium tert-butoxide were added to 155 ml of toluene and dissolved in it. Next, 0.178 g (0.31 mmol) of Pd(dba) 2 and 0.125 g (0.62 mmol) of tri-tert-butylphosphine were sequentially added, and then refluxed and stirred under a nitrogen atmosphere for 4 hours. When the reaction was terminated, the resultant was extracted with toluene and distilled water, and the organic layer was dried over magnesium sulfate under reduced pressure and filtered and concentrated. The product was purified by silica gel column chromatography using n-hexane / dichloromethane (8:2 volume ratio) to obtain 16 g (92% yield) of the target compound F-94.
[1291] LC-mass spectrum (theoretical: 563.22 g / mol, measured: M+=563.28 g / mol)
example 2
[1292] Example 2: Preparation of compound G-94
[1293]
[1294] In a round bottom flask, 10.5 g (30.9 mmol) of intermediate M-2, 9.9 g (30.9 mmol) of bis(4-benzidine)amine and 4.5 g (46.35 mmol) of sodium tert-butoxide were added to 155 ml of toluene and dissolved in it. Next, 0.178 g (0.31 mmol) of Pd(dba) 2 and 0.125 g (0.62 mmol) of tri-tert-butylphosphine were sequentially added, and then refluxed and stirred under a nitrogen atmosphere for 4 hours. When the reaction was terminated, the resultant was extracted with toluene and distilled water, and the organic layer was dried over magnesium sulfate under reduced pressure and filtered and concentrated. The product was purified by silica gel column chromatography using n-hexane / dichloromethane (8:2 volume ratio) to obtain 16.8 g (94% yield) of the target compound G-94.
[1295] LC-mass spectrum (theoretical: 579.20 g / mol, measured: M+=579.32 g / mol)
example 3
[1296] Example 3: Preparation of Compound F-99
[1297]
[1298] In a round bottom flask, 10 g (30.9 mmol) of intermediate M-1, 11.2 g (30.9 mmol) of biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl )-amine and 4.5 g (46.35 mmol) of sodium tert-butoxide were added to and dissolved in 155 ml of toluene. Next, 0.178 g (0.31 mmol) of Pd(dba) 2 and 0.125 g (0.62 mmol) of tri-tert-butylphosphine were sequentially added, and then refluxed and stirred under a nitrogen atmosphere for 4 hours. When the reaction was terminated, the resultant was extracted with toluene and distilled water, and the organic layer was dried over magnesium sulfate under reduced pressure and filtered and concentrated. The product was purified by silica gel column chromatography using n-hexane / dichloromethane (8:2 volume ratio) to obtain 17.2 g (92% yield) of the target compound F-99.
[1299] LC-mass spectrum (theoretical: 603.26 g / mol, measured: M+=603.31 g / mol)
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com