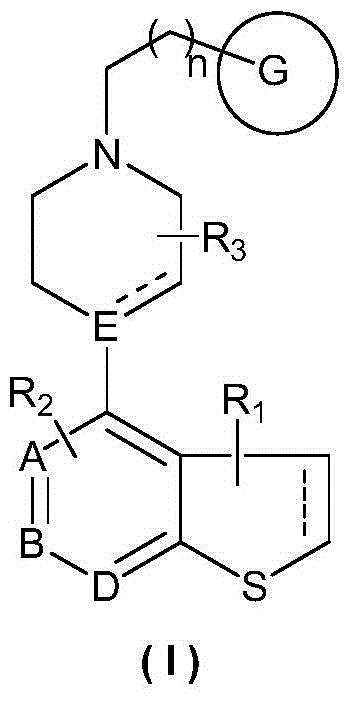
Heterocyclic compound, preparation method therefor and use thereof
A technology of heterocyclic compounds and heterocyclic groups, which is applied in the application field of preparing medicines for diseases of the central nervous system, and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0271] Preparation Example 1 Preparation of 1-(benzothiophen-4-yl)piperazine hydrochloride
[0272]
[0273] step 1:
[0274] Dissolve 2-chloro-6-fluorobenzaldehyde 1-a (500mg, 3.15mmol), N-tert-butoxycarbonylpiperazine (646mg, 3.47mmol) into N,N-dimethylformamide (5ml), Under nitrogen protection, potassium carbonate (2.18g, 15.77mmol) was added at room temperature, the mixture was stirred at 80°C for 4 hours, cooled and filtered, water (20ml) was added, extracted with ethyl acetate (5ml×3), and 1N hydrochloric acid (3ml) Washing, washing with saturated aqueous sodium bicarbonate, drying over anhydrous sodium sulfate, and concentrating to obtain a solid, which was slurried with petroleum ether (50ml) for 1 hour, and filtered to obtain a pale yellow solid 1-b (1.0g, yield: 90%). 1 H-NMR (300Hz, DMSO-d 6 ): δppm 10.19(s,1H),7.52(t,1H),7.18(d,2H),3.46(t,4H),2.94(t,4H),1.39(s,9H).
[0275] Step 2:
[0276] in N 2 Under protection, compound 1-b (5g, 15.3mmol), ethyl thiogly...
preparation example 2
[0283] Preparation Example 2 Preparation of 5-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)pentyl methanesulfonate
[0284]
[0285] step 1:
[0286] Drop into 7-hydroxyl-3,4-dihydroquinolin-2(1H)-one 2-a (10g, 61.3mmol) in reaction bottle, add chloroform (100ml) and pyridine (10.6g, 134mmol), room temperature Stir for 10 minutes, cool down to below 0°C, slowly add trifluoromethanesulfonic anhydride (17.2g, 60.99mmol) dropwise, stir for 30 minutes, remove the ice bath, react at room temperature for 1 hour, filter out the solid, and wash with 1M hydrogen sulfate Washed twice with potassium aqueous solution and water, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave light yellow solid 2-b (12 g, yield: 67%).
[0287] Step 2:
[0288] Compound 2-b (18g, 61.0mmol), bis(triphenylphosphine)palladium dichloride (3.6g, 5.12mmol) and cuprous iodide (3.96g, 20.8mmol) were put into the reaction flask, and replaced with nitrogen three times, Inject 4-penty...
preparation example 3
[0293] Preparation Example 3 Preparation of 5-(2-oxo-1,2-dihydroquinolin-7-yl)pentyl methanesulfonate
[0294]
[0295] Take the product of Preparation Example 2 (0.8g, 2.57mmol) and add 1,4-dioxane (5ml), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.875mg, 3.85mmol), heated at 95°C for 3 hours, cooled the reaction solution, filtered to remove insoluble matter, added dichloromethane to the filtrate, washed with saturated sodium bicarbonate solution, sodium thiosulfate solution and saturated brine successively, anhydrous sodium sulfate Drying, concentration, and column chromatography gave a white solid (0.6 g, yield: 75%). ESI-MS(m / z):310.1[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com