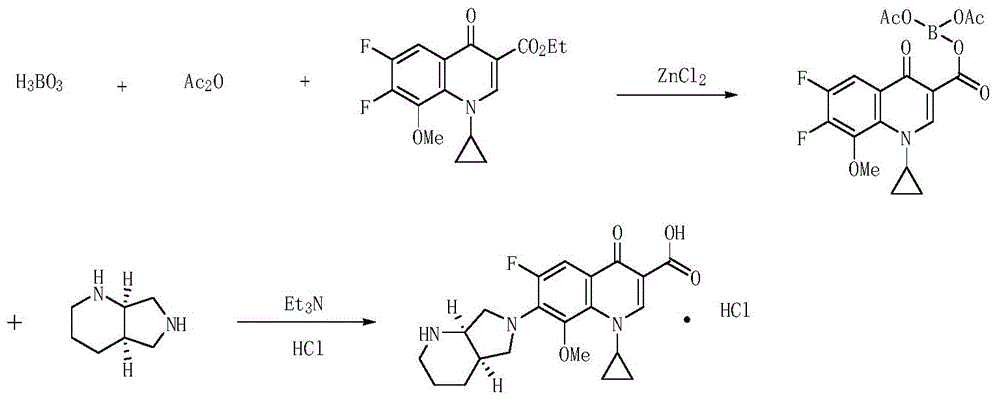
Production method of moxifloxacin hydrochloride
A technology for moxifloxacin hydrochloride and its production method, which is applied in the production field of moxifloxacin hydrochloride, can solve the problems of being unsuitable for large-scale industrial production, difficult nucleophilic substitution reaction, and low electron cloud density, and is easy for industrial production and recycling. Convenience and low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] 2) Preparation of moxifloxacin hydrochloride: Add 800g of acetonitrile, 160g of chelate, and 50g of triethylamine into the reaction flask, and drop (S,S)-2,8-diazacyclobicyclo[4, 3,0]nonane in acetonitrile solution ((S,S)-2,8-diazacyclobicyclo[4,3,0]nonane 47g, acetonitrile 100g), after the dropwise addition, react at 25°C for 3 hour, the reaction is complete. Turn on the vacuum pump and concentrate under reduced pressure (vacuum degree -0.04~-0.09MPa). After concentration, add 800g of ethanol, stir to dissolve, add concentrated hydrochloric acid dropwise at a temperature of 25°C, adjust to pH=2, and crystallize at 25°C for 3 hours. Filter, wash with ethanol, and dry under normal pressure at 60°C to obtain 125 g of yellow crystalline solid, yield 75.5%, specific rotation [a] D 20 -126° (standard is -125° to -138°).
[0052] The second embodiment:
[0053] 1) Preparation of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate chelate: t...
experiment example
[0068] In order to investigate the quality of the moxifloxacin hydrochloride produced by the present invention, we have carried out a large number of comparative experiments with various processes, all of which prove that the present invention has advantages in quality or production cost. Especially compared with the relatively close chelation process, positive results have been obtained. Here is just one example:
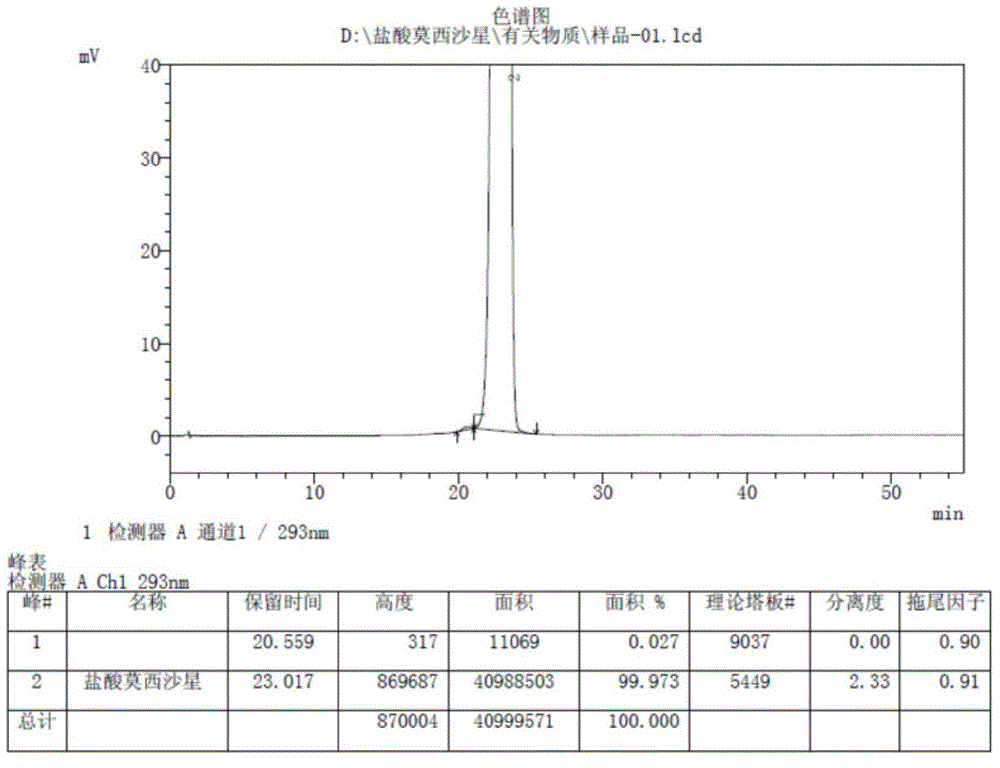
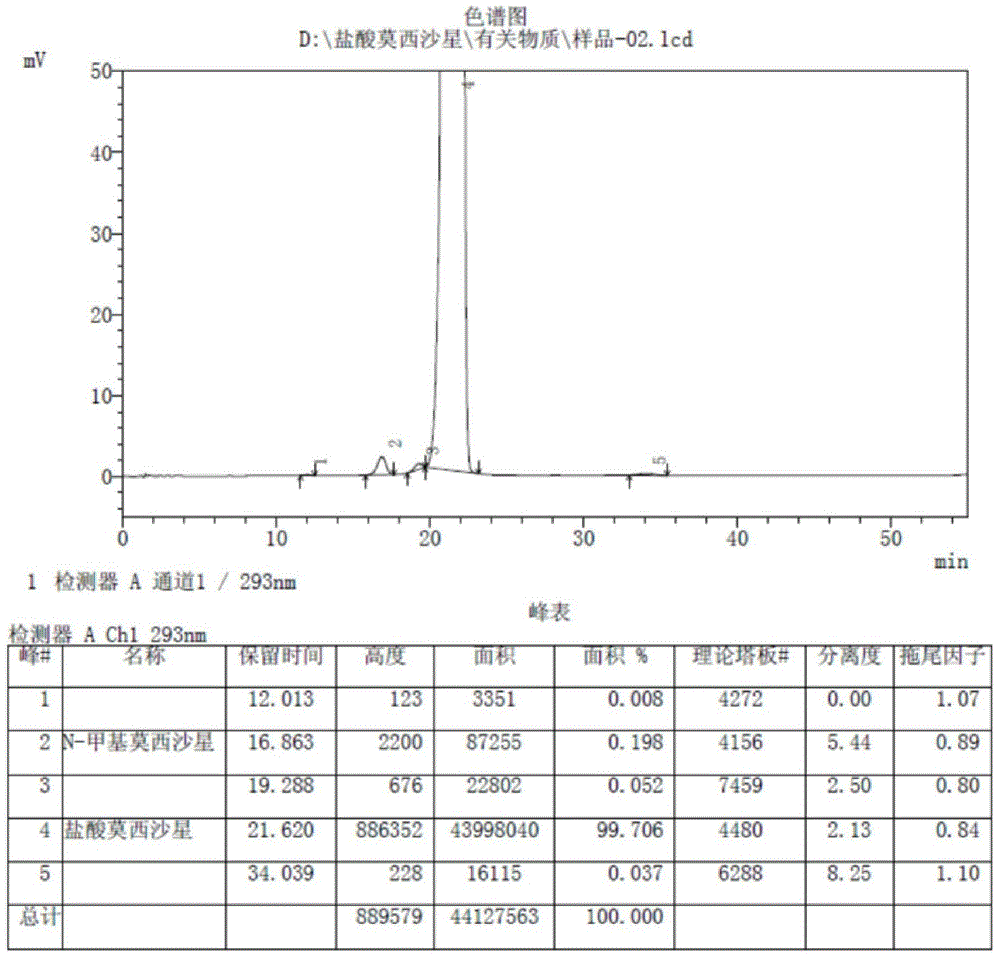
[0069] The moxifloxacin hydrochloride product of the present invention is compared with the moxifloxacin hydrochloride product of the patent WO2005012285. Analyze by high performance liquid chromatography (HPLC), obtain the description of the accompanying drawings figure 2 and image 3 . in, figure 2 It is the moxifloxacin hydrochloride HPLC spectrogram produced by the present invention; Its experimental data are as table 1:
[0070] Table 1
[0071] Peak table
[0072] Detector A Ch1 293nm
[0073] Peak#
[0074] image 3 It is the HPLC spectr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com