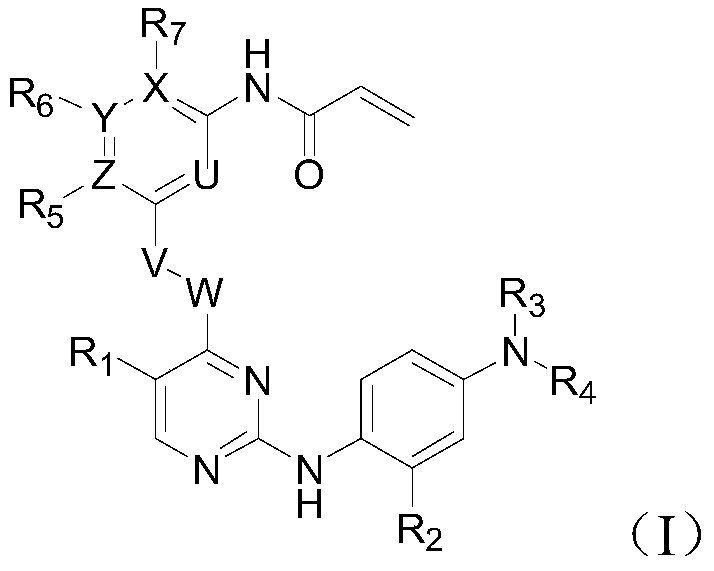
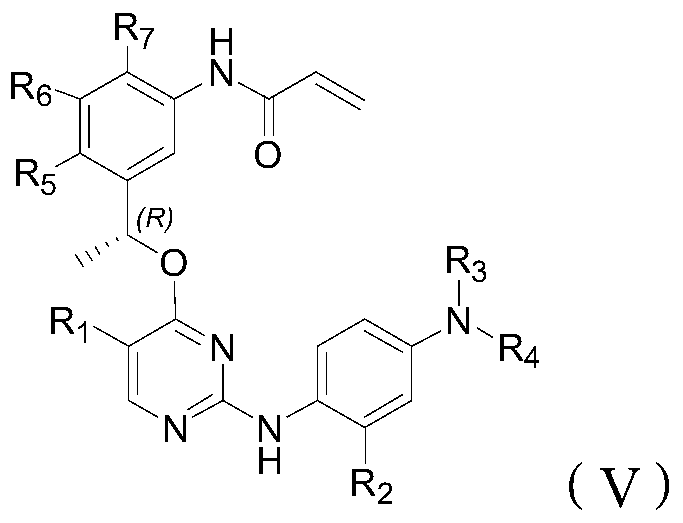
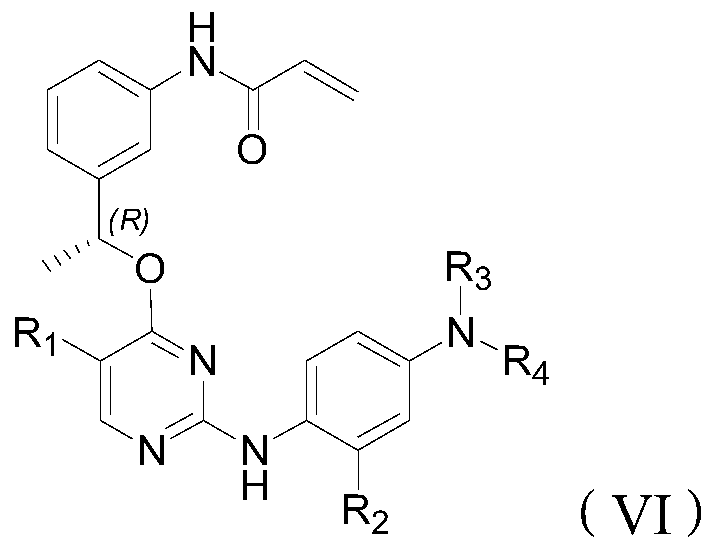
Inhibitor of t790m mutant egfr and its application in the preparation of antitumor drugs
A technology of anti-tumor drugs and inhibitors, which is applied in the field of drugs for the treatment of tumors, and can solve the problems of poor selectivity, hindering the combination of inhibitors and ATP binding regions, and reducing the affinity of inhibitors and EGFR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1, (R)-N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-tri Fluoromethylpyrimidin-4-yl)oxy)ethyl)propyl)acrylamide
[0091]
[0092] Synthetic steps:
[0093]
[0094] Step 1: Preparation of tert-butyl 3-acetylphenylcarbamate (compound 2)
[0095] To a solution of 1-(3-aminophenyl)ethanone (compound 1) (8.0 g, 59.3 mmol) in dioxane (100 mL) was added (Boc) 2 O (16.8 g, 77.1 mmol). The obtained reaction solution was reacted at 150° C. for 4 hours, and then concentrated under reduced pressure. The residue was subjected to silica gel column chromatography (PE:EA=8:1 to 4:1) to obtain the target product (12.4 g, 88.6% yield) as a white solid. 1 H NMR (400MHz, CDCl 3 )δ7.95(s,1H),7.66(d,J=7.6Hz,1H),7.60(d,J=7.8Hz,1H),7.37(t,J=7.9Hz,1H),6.88(s, 1H), 2.59(s, 3H), 1.52(s, 9H).
[0096] Step 2: Preparation of tert-butyl (R)-(3-(1-hydroxyethyl)phenyl)carbamate (Compound 3)
[0097] (+)-DIP-Cl (5.5 g, 17.0 mmol) was dissolved in anhydrous THF...
Embodiment 2
[0104] Example 2, (S)-N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-tri Fluoromethylpyrimidin-4-yl)oxy)ethyl)propyl)acrylamide
[0105]
[0106] The synthetic method is as embodiment 1,
[0107] 1 H NMR (400MHz, CDCl 3 )δ8.34(s,1H),7.86(m,2H),7.57(m,3H),7.30(t,J=7.8Hz,1H),7.16(d,J=7.4Hz,1H),6.64– 6.37(m,3H),6.28(m,2H),5.78(d,J=10.3Hz,1H),3.87(s,3H),3.80–3.70(m,2H),3.69–3.54(m,2H) ,3.22–2.98(m,4H),2.19(s,3H),1.66(d,J=6.6Hz,3H).LC-MS:m / z 585(M+H) + .
Embodiment 3
[0108] Example 3, N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-trifluoromethylpyrimidine -4-yl)oxy)ethyl)propyl)acrylamide
[0109]
[0110] The synthetic method is as embodiment 1,
[0111] 1 H NMR (400MHz, CDCl 3 )δ8.34(s,1H),7.85(m,2H),7.48(m,3H),7.31(t,J=7.8Hz,1H),7.16(d,J=7.6Hz,1H),6.64– 6.37(m,3H),6.27(m,2H),5.79(d,J=10.2Hz,1H),3.86(s,3H),3.82–3.70(m,2H),3.68–3.58(m,2H) ,3.25–2.91(m,4H),2.06(s,3H),1.66(d,J=6.5Hz,3H).LC-MS:m / z 585(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com