Lesinurad analog and preparation method and medical application thereof
A technology of analogs and medicinal salts, applied in the field of medicinal chemistry, can solve the problems of slow development, difficulty in developing therapeutically effective compounds, and no great progress, and achieve low toxic and side effects, significant curative effect, and high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
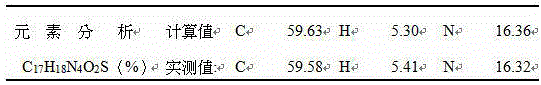
[0046] Embodiment 1: 2-(1-(4-cyclopropylnaphthalene-1-yl)-1H-tetrazolium-5-sulfur)acetic acid (compound I 1 ) preparation
[0047] Preparation process:
[0048]
[0049] 1) Preparation of 1-cyclopropylnaphthalene
[0050] To a dry 5 L round bottom flask, add 1-bromonaphthalene (414.0 g, 2 mol), [1,3-bis(diphenylphosphino)propane]nickel chloride (165 g, 0.4 mol) and 1500 mL of dry 3000 mL of THF solution of 1.0 mol / L cyclopropylmagnesium bromide was slowly added dropwise to the system with a constant pressure dropping funnel. After the dropwise addition, the reaction compound was stirred at room temperature for 8 h under nitrogen protection, then heated to reflux, and reacted for 36 h. After the reaction was complete, the reaction mixture was cooled to room temperature, then carefully poured into 10 L of stirred ice water, stirred, and adjusted to pH 2 with concentrated hydrochloric acid, CH 2 Cl 2(4000 mL×3) extraction. Combine the organic phases, wash with 3000 mL of...
Embodiment 2
[0068] Embodiment 2: 2-(1-(4-cyclopropyl naphthalene-1-yl)-1H-tetrazolium-5-sulfur) sodium acetate (compound I 1 ) Preparation of sodium salt
[0069] In a dry reaction kettle of 5L, add compound I 1 (162g, 0.49mol) and 2500mL methanol, stirred at room temperature, added 300mL of NaOH (19.6g, 0.49mol) aqueous solution, stirred at room temperature for 20min, then evaporated to dryness in a rotary evaporator, and the residue was vacuum-dried to obtain a light yellow amorphous solid , the above solid was recrystallized with 95% ethanol to obtain white solid compound I 1 Sodium salt 138g, yield 81%, HPLC content 99.6%.
Embodiment 3
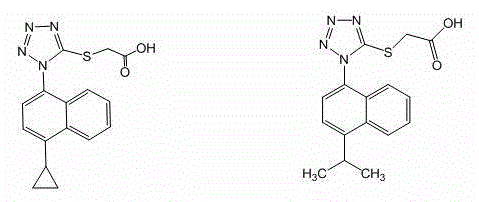
[0070] Embodiment 3: Compound I 2 preparation of
[0071] The target compound was synthesized in the same manner as in Example 1, substituting isopropylmagnesium bromide for cyclopropylmagnesium bromide.
[0072]
[0073] 1 H—NMR (500MHz, CDCl 3 / TMS,ppm):
[0074] 12.91 (1H, bs, CO 2 H), 8.50 (1H, d, J = 9.7 Hz, Ar-H), 7.55 ~ 7.66 (4H, m, Ar-H), 7.43 (d, 1H, J = 9.7 Hz, Ar-H), 7.15 ( 1H, d, J=11.6Hz, Ar-H), 3.99 (s, 2H, SCH 2 ), 2.47~2.52 (m, 1H, isopropyl-CH), 1.42 (s, 6H, isopropyl-CH 3 ).
[0075] MS: m / z (M + ) 328 (100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com