Organic positive electrode active material as well as preparation method and application of organic positive electrode active material
A positive electrode active material and organic technology, applied in the field of organic positive electrode active materials and their preparation, can solve the problems of poor cycle stability of materials and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment prepares the organic cathode active material, comprising the following steps:
[0044] Weigh 4mmol of 2,6-diaminoanthraquinone and 4mmol of pyromellitic dianhydride, respectively, and transfer them to a 50mL two-necked flask. Add 30mL of m-cresol and 10 drops (about 1ml) of isoquinoline, put it into a magnetic stirrer, connect the condenser tube and the double-row tube, and connect the side tube to the liquid-sealed bubbler. Warm up to 50°C under argon atmosphere and stir for a while. Then the temperature was raised to 100° C. for 10 hours, and then the temperature was raised to 200° C. for 12 hours. The moisture generated during the reaction was discharged into the fume hood through the liquid-sealed bubbler. After the reaction was completed, it was cooled to room temperature, and the mixture was poured into methanol for precipitation. The product was washed with methanol and hot water, and dried in vacuum before use.
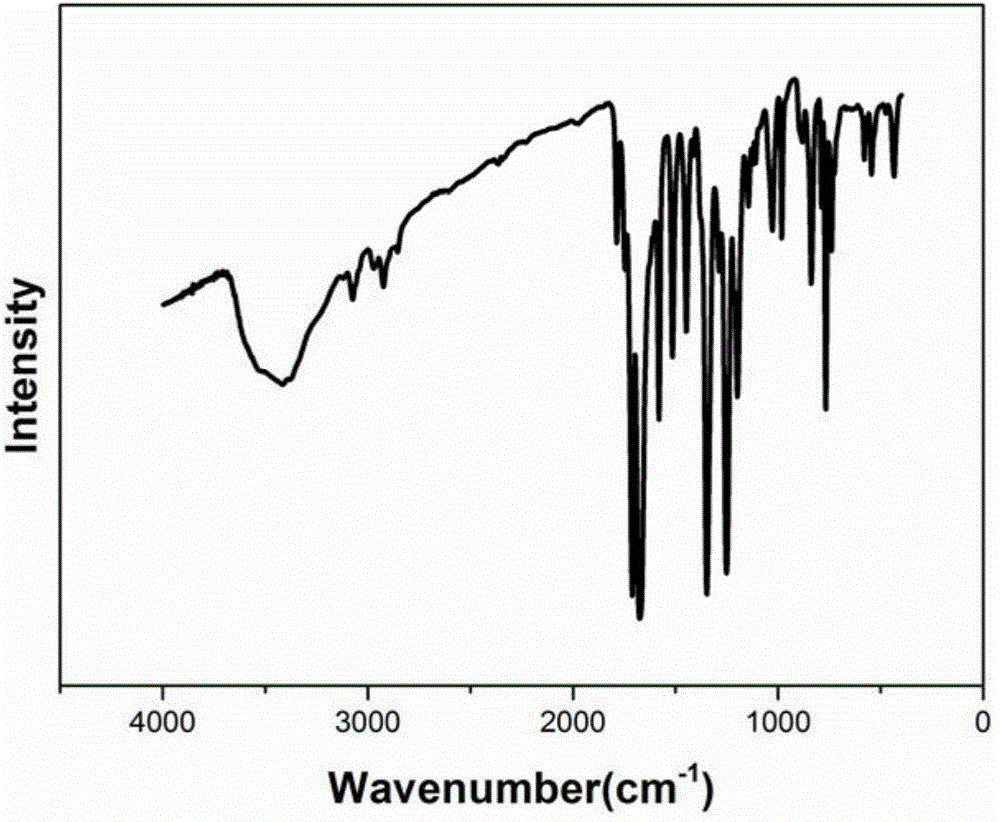
[0045] The infrared spectrum ...
Embodiment 2
[0049] This embodiment prepares the organic cathode active material, comprising the following steps:
[0050] Weigh 3 mmol of p-2,7-diaminoanthraquinone and 3 mmol of naphthalene tetracarboxylic dianhydride, respectively, and transfer them to a 50 mL two-necked flask. Add 30 mL of m-cresol and 10 drops of isoquinoline, put in a magnetic stirring bar, connect the condenser tube and the double-row tube, and connect the side tube to the liquid-sealed bubbler. Warm up to 50°C under argon atmosphere and stir for a while. Then the temperature was raised to 100° C. for 10 hours, and then the temperature was raised to 200° C. for 12 hours. The moisture generated during the reaction was discharged into the fume hood through the liquid-sealed bubbler. After the reaction was completed, it was cooled to room temperature, and the mixture was poured into methanol for precipitation. The product was washed with methanol and hot water, and dried in vacuum before use.
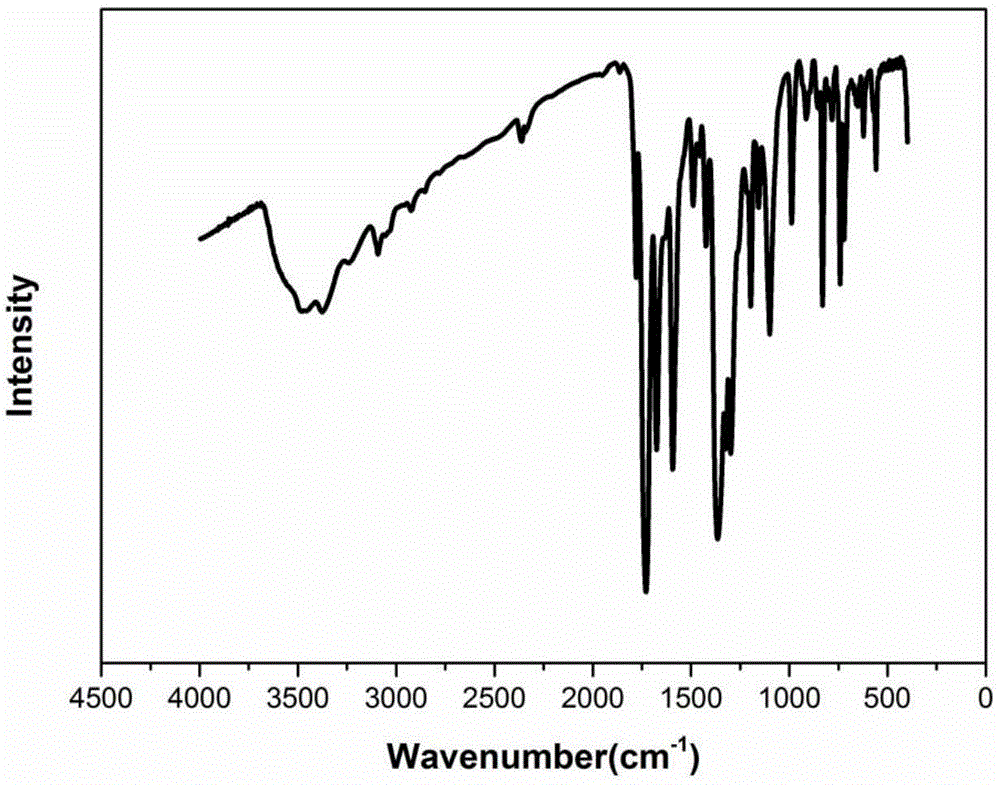
[0051] The infrared ...
Embodiment 3
[0054] This embodiment prepares the organic cathode active material, comprising the following steps:
[0055] Weigh 4.5mmol 2,6-diaminoanthraquinone and 4.5mmol isophthalamide respectively, and transfer them to a 50mL two-necked flask. Add 30 mL of m-cresol and 10 drops of isoquinoline, put in a magnetic stirring bar, connect the condenser tube and the double-row tube, and connect the side tube to the liquid-sealed bubbler. Warm up to 50°C under argon atmosphere and stir for a while. Then the temperature was raised to 100° C. for 10 hours, and then the temperature was raised to 200° C. for 12 hours. The moisture generated during the reaction was discharged into the fume hood through the liquid-sealed bubbler. After the reaction was completed, it was cooled to room temperature, and the mixture was poured into methanol for precipitation. The product was washed with methanol and hot water, and dried in vacuum before use.
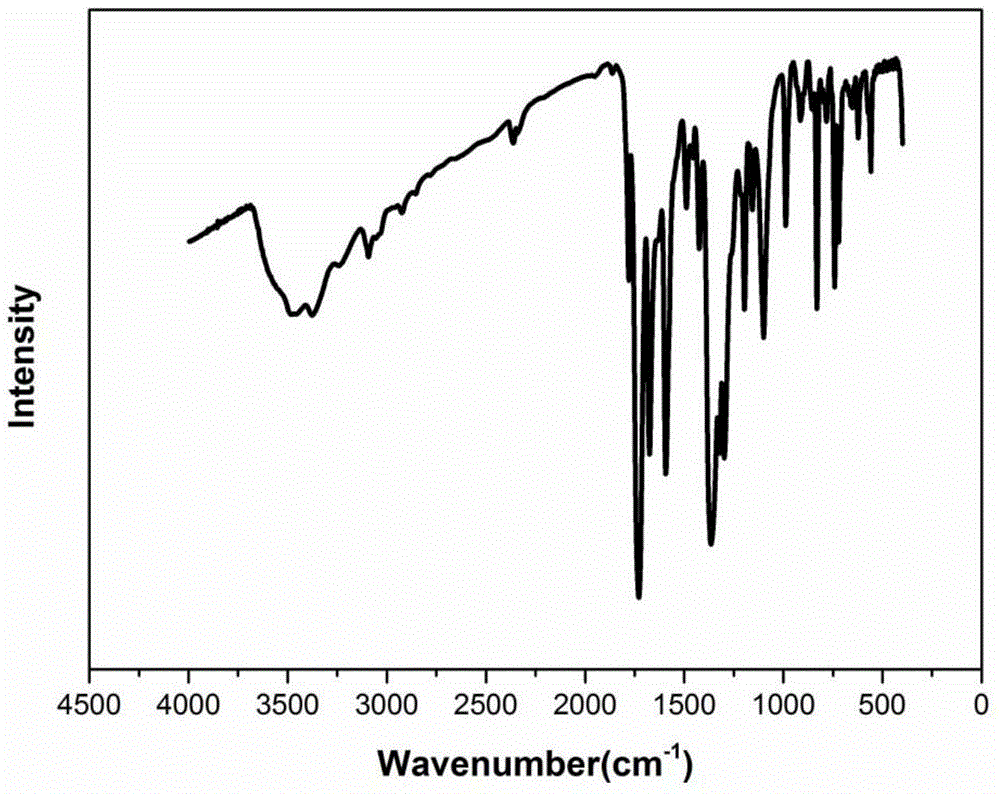
[0056] The infrared spectrum of the prepared organic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com